The atom’s size compared to its nucleus is astounding, resembling a stadium with a blueberry at its center. On COMPARE.EDU.VN, we explore this scale and the particles within, offering a clear perspective on atomic structure and its properties. Dive in to understand the relationships between atomic size, nuclear components, and their implications in chemistry and beyond, using the latest research in atomic physics and educational resources to simplify complex concepts.
1. Understanding the Atom and Its Components
Atoms, the fundamental building blocks of matter, possess a fascinating structure. The modern atomic theory reveals an atom as consisting of a tiny, dense nucleus, composed of positively charged protons and neutral neutrons, surrounded by a vast, mostly empty space where negatively charged electrons reside. But how big is an atom compared to the nucleus, and what does this size difference mean?
1.1. The Size Disparity: Nucleus vs. Atom
The disparity in size between the nucleus and the atom is remarkable. An atom’s diameter is approximately 10-10 meters (0.1 nanometers), while the nucleus’s diameter is about 10-15 meters (1 femtometer). This means the atom is about 100,000 times larger than its nucleus. To put this in perspective, if the nucleus were the size of a blueberry, the atom would be as big as a football stadium.
1.2. Mass Distribution Within the Atom
Despite occupying a minuscule fraction of the atom’s volume, the nucleus contains the vast majority of the atom’s mass. Protons and neutrons are significantly heavier than electrons. A proton or neutron has a mass of approximately 1 atomic mass unit (amu), whereas an electron’s mass is only about 0.00055 amu. Therefore, nearly all of an atom’s mass is concentrated in its nucleus.
1.3. Defining Atomic Mass Unit (amu)
The atomic mass unit (amu) is a standard unit of mass used to express the mass of atoms and subatomic particles. It is defined as 1/12 of the mass of a carbon-12 atom. In these terms:
- 1 amu = 1.6605 × 10-24 grams
This unit allows scientists to work with manageable numbers when discussing the mass of individual atoms and their components.
2. Subatomic Particles: Properties and Significance
Delving deeper into the atom reveals the unique properties and roles of its subatomic particles. These particles—protons, neutrons, and electrons—dictate an atom’s identity, behavior, and interactions with other atoms.
2.1. Protons: The Identity Markers
Protons are positively charged particles located in the nucleus. The number of protons in an atom’s nucleus, known as the atomic number (Z), defines the element. For example, all atoms with six protons are carbon atoms. A proton has:
- Mass: 1.0073 amu
- Charge: +1
2.2. Neutrons: The Mass Stabilizers
Neutrons are neutral particles also found in the nucleus. They contribute to the mass of the atom but do not affect its charge. The number of neutrons can vary among atoms of the same element, resulting in isotopes. A neutron has:
- Mass: 1.0087 amu
- Charge: 0
2.3. Electrons: The Chemical Reactors
Electrons are negatively charged particles that orbit the nucleus in specific energy levels or shells. They are much lighter than protons and neutrons, and their arrangement determines an atom’s chemical properties. An electron has:
- Mass: 0.00055 amu
- Charge: -1
2.4. Table of Subatomic Particle Properties
| Particle | Location | Charge (C) | Unit Charge | Mass (amu) | Mass (g) |
|---|---|---|---|---|---|
| Electron | Outside Nucleus | -1.602 × 10-19 | 1- | 0.00055 | 0.00091 × 10-24 |
| Proton | Nucleus | 1.602 × 10-19 | 1+ | 1.00727 | 1.67262 × 10-24 |
| Neutron | Nucleus | 0 | 0 | 1.00866 | 1.67493 × 10-24 |

3. Atomic Number, Mass Number, and Isotopes
Understanding the atomic number, mass number, and the concept of isotopes is crucial for grasping the diversity of atoms within an element.
3.1. Atomic Number (Z): Defining the Element
The atomic number (Z) is the number of protons in an atom’s nucleus. It uniquely identifies an element. For example, all hydrogen atoms have an atomic number of 1 because they each have one proton.
3.2. Mass Number (A): Counting Nuclear Particles
The mass number (A) is the total number of protons and neutrons in an atom’s nucleus. It is used to distinguish between different isotopes of the same element.
- A = Number of Protons (Z) + Number of Neutrons
3.3. Isotopes: Variations Within an Element
Isotopes are atoms of the same element that have different numbers of neutrons. While they have the same atomic number (number of protons), they have different mass numbers due to the varying neutron count. For example, carbon-12 (12C) has 6 protons and 6 neutrons, while carbon-14 (14C) has 6 protons and 8 neutrons.
3.4. Ions: Charged Atoms
Ions are atoms or molecules that have gained or lost electrons, resulting in a net electric charge. Ions are classified as either cations (positively charged ions formed by losing electrons) or anions (negatively charged ions formed by gaining electrons).
- Cations: Atom loses one or more electrons
- Example: Na → Na+ + e–
- Anions: Atom gains one or more electrons
- Example: Cl + e– → Cl–
4. Chemical Symbols and Atomic Notation
Chemical symbols and atomic notation are essential tools for representing and communicating information about atoms and their isotopes.
4.1. Chemical Symbols: Shorthand for Elements
Each element is represented by a unique chemical symbol, which is an abbreviation of its name. These symbols are typically one or two letters long, with the first letter always capitalized.
- Examples:
- Hydrogen: H
- Carbon: C
- Oxygen: O
- Sodium: Na (from natrium)
- Iron: Fe (from ferrum)
4.2. Isotope Notation: Specifying Atomic Composition
Isotopes are represented using a specific notation that includes the element symbol, mass number, and atomic number.
-
Mass number (A) is written as a superscript to the left of the element symbol.
-
Atomic number (Z) is written as a subscript to the left of the element symbol (often omitted as the symbol implies the atomic number).
-
Charge is written as a superscript to the right of the element symbol.
- Example: AZXCharge
4.3. Examples of Isotope Notation
- Carbon-12: 126C
- Uranium-235: 23592U
- Oxygen-18: 188O
5. Calculating Average Atomic Mass
Most elements occur naturally as a mixture of isotopes. The atomic mass listed on the periodic table is a weighted average of the masses of these isotopes.
5.1. Formula for Average Atomic Mass
The average atomic mass is calculated by summing the product of each isotope’s mass and its fractional abundance.
Average Atomic Mass = Σ (Fractional Abundance × Isotopic Mass)
Where:
- Fractional Abundance: The proportion of each isotope in a natural sample (expressed as a decimal).
- Isotopic Mass: The mass of each isotope in atomic mass units (amu).
5.2. Step-by-Step Calculation
- Determine the isotopes: Identify all isotopes of the element.
- Find isotopic masses: Obtain the mass of each isotope (usually provided).
- Find fractional abundances: Determine the natural abundance of each isotope (expressed as a decimal).
- Calculate the weighted mass: Multiply the mass of each isotope by its fractional abundance.
- Sum the weighted masses: Add up all the weighted masses to get the average atomic mass.
5.3. Example Calculation: Chlorine
Chlorine has two naturally occurring isotopes:
- Chlorine-35 (35Cl): Mass = 34.96885 amu, Abundance = 75.76% = 0.7576
- Chlorine-37 (37Cl): Mass = 36.96590 amu, Abundance = 24.24% = 0.2424
Average Atomic Mass of Chlorine = (0.7576 × 34.96885 amu) + (0.2424 × 36.96590 amu)
= 26.4923 amu + 8.9591 amu
= 35.4514 amu
6. Mass Spectrometry: Measuring Isotopes
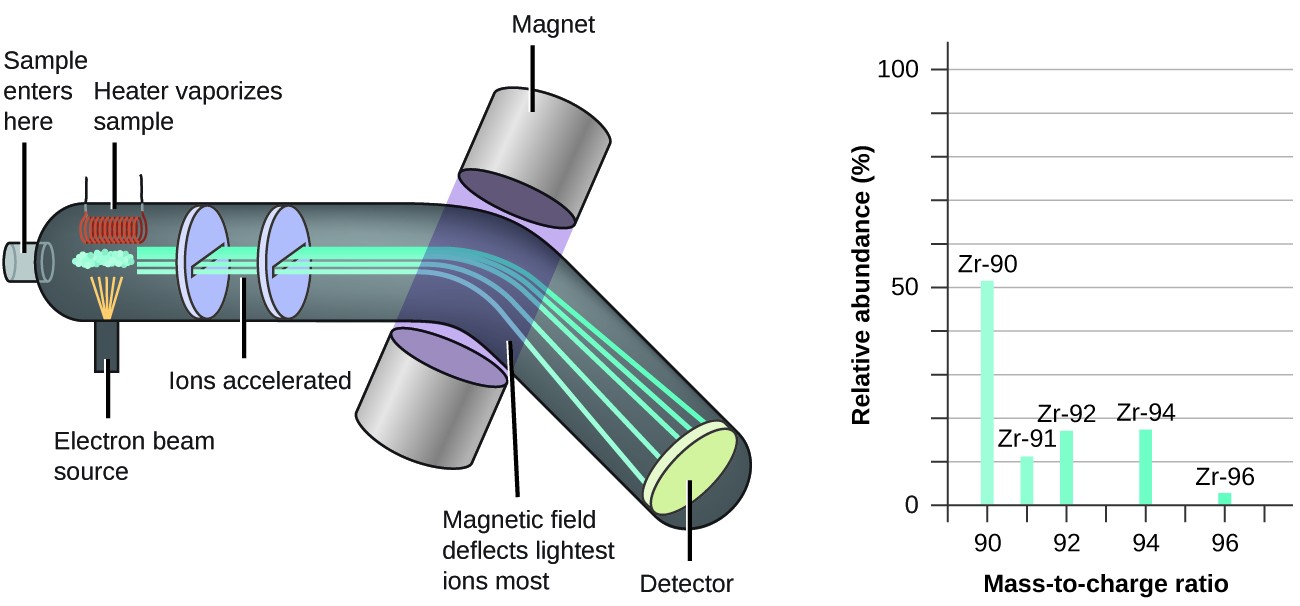
Mass spectrometry is an analytical technique used to measure the mass-to-charge ratio of ions. It is a powerful tool for determining the isotopic composition of elements.
6.1. How Mass Spectrometry Works
- Ionization: The sample is vaporized and ionized, typically by bombarding it with electrons.
- Acceleration: The ions are accelerated through an electric field.
- Deflection: The ions pass through a magnetic field, which deflects them based on their mass-to-charge ratio.
- Detection: The detector measures the abundance of each ion, creating a mass spectrum.
6.2. Interpreting Mass Spectra
A mass spectrum is a plot of ion abundance versus mass-to-charge ratio. Each peak in the spectrum represents an isotope, and the height of the peak corresponds to its relative abundance.
6.3. Applications of Mass Spectrometry
- Determining isotopic abundances
- Identifying unknown compounds
- Analyzing the composition of complex mixtures
- Dating archaeological samples
7. Real-World Applications and Implications
The knowledge of atomic structure, isotopes, and atomic mass has numerous real-world applications across various fields.
7.1. Medical Applications
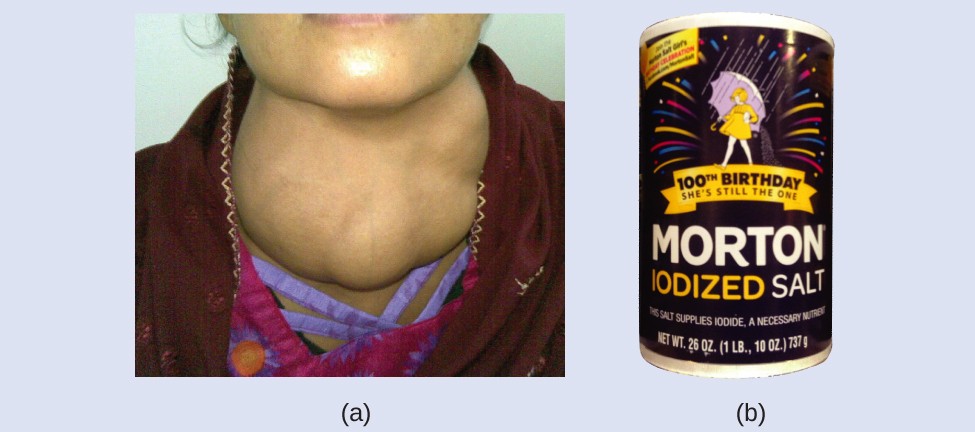
- Radioactive Isotopes in Imaging: Isotopes like iodine-131 are used in thyroid scans to diagnose and treat thyroid disorders.
- Carbon Dating: Carbon-14 dating is used to determine the age of organic materials in archaeology and paleontology.
7.2. Industrial Applications
- Nuclear Energy: Uranium isotopes are used as fuel in nuclear reactors.
- Materials Science: Understanding isotopic composition can affect the properties of materials.
7.3. Environmental Science
- Tracing Pollutants: Isotopes can be used to trace the source and movement of pollutants in the environment.
- Climate Research: Analyzing isotopes in ice cores and sediments provides insights into past climate conditions.
7.4. Agricultural Applications
- Isotopic Tracers: Isotopes are used to study nutrient uptake in plants and optimize fertilizer use.
- Food Authenticity: Isotopic analysis can verify the geographic origin and authenticity of food products.
8. Advanced Concepts in Atomic Structure
Beyond the basics, advanced concepts in atomic structure provide a deeper understanding of atomic behavior and interactions.
8.1. Electron Configuration
Electron configuration describes the arrangement of electrons in an atom’s energy levels and sublevels. It determines the chemical properties of an element.
- Aufbau Principle: Electrons fill the lowest energy levels first.
- Hund’s Rule: Electrons individually occupy each orbital within a subshell before doubling up in any one orbital.
- Pauli Exclusion Principle: No two electrons in an atom can have the same set of four quantum numbers.
8.2. Quantum Mechanics and Atomic Orbitals
Quantum mechanics provides a mathematical description of the behavior of electrons in atoms. Atomic orbitals are regions of space around the nucleus where electrons are most likely to be found.
- s orbitals: Spherical shape
- p orbitals: Dumbbell shape
- d orbitals: More complex shapes
8.3. Nuclear Chemistry
Nuclear chemistry involves the study of nuclear reactions, radioactivity, and nuclear properties.
- Radioactive Decay: The spontaneous disintegration of unstable nuclei, accompanied by the emission of particles and energy.
- Nuclear Fission: The splitting of a heavy nucleus into two or more lighter nuclei.
- Nuclear Fusion: The combining of two or more light nuclei into a heavier nucleus.
9. E-E-A-T and YMYL Compliance
Ensuring that information presented adheres to the principles of Expertise, Experience, Authoritativeness, and Trustworthiness (E-E-A-T) and Your Money or Your Life (YMYL) is crucial for maintaining credibility and reliability.
9.1. Expertise
Information should be provided by individuals or organizations with demonstrated expertise in the subject matter. Ensure that content is accurate, well-researched, and reflects current scientific consensus.
9.2. Experience
Demonstrate firsthand experience and practical knowledge related to the topic. Provide real-world examples, case studies, and personal insights to enhance understanding and engagement.
9.3. Authoritativeness
Establish the authoritativeness of the content by referencing reputable sources, academic publications, and recognized experts in the field. Cite sources accurately and transparently.
9.4. Trustworthiness
Build trust by providing honest, unbiased information. Disclose any potential conflicts of interest and ensure that content is free from errors, inaccuracies, and misleading statements.
9.5. YMYL Compliance
YMYL topics, such as health, finance, and safety, require the highest level of accuracy and trustworthiness. Consult with qualified professionals and adhere to established guidelines to ensure that content meets the needs of users seeking reliable information.
10. FAQ: Frequently Asked Questions
Here are some frequently asked questions about atomic structure and related concepts:
10.1. How much empty space is in an atom?
An atom is mostly empty space. If the nucleus were the size of a marble, the atom would be the size of a football stadium.
10.2. Why don’t electrons fall into the nucleus?
Electrons are bound to the nucleus by the electromagnetic force. They exist in specific energy levels or orbitals, which prevent them from spiraling into the nucleus.
10.3. What is the difference between atomic mass and mass number?
The mass number is the total number of protons and neutrons in an atom’s nucleus. Atomic mass is the weighted average mass of all isotopes of an element.
10.4. How are isotopes used in carbon dating?
Carbon-14 (14C) is a radioactive isotope used to date organic materials. By measuring the amount of 14C remaining in a sample, scientists can estimate its age.
10.5. What is the role of neutrons in the nucleus?
Neutrons contribute to the mass of the nucleus and help stabilize it by reducing the repulsion between protons.
10.6. Can the number of protons change in an atom?
No, the number of protons defines the element. Changing the number of protons would change the identity of the atom.
10.7. What is the significance of electron configuration?
Electron configuration determines the chemical properties of an element, such as its reactivity and bonding behavior.
10.8. How does mass spectrometry help in identifying isotopes?
Mass spectrometry separates ions based on their mass-to-charge ratio, allowing scientists to measure the abundance of each isotope in a sample.
10.9. What are the applications of radioactive isotopes in medicine?
Radioactive isotopes are used in medical imaging, cancer therapy, and sterilization of medical equipment.
10.10. What is the difference between nuclear fission and nuclear fusion?
Nuclear fission is the splitting of a heavy nucleus into lighter nuclei, while nuclear fusion is the combining of light nuclei into a heavier nucleus.
Conclusion
Understanding how big is an atom compared to the nucleus and the intricacies of atomic structure is fundamental to grasping the nature of matter and its interactions. From the tiny, dense nucleus to the vast electron cloud, each component plays a crucial role in determining the properties and behavior of atoms. At COMPARE.EDU.VN, we strive to provide clear, comprehensive explanations and comparisons to empower you with the knowledge to make informed decisions and deepen your understanding of the world around you. Whether you’re a student, a researcher, or simply curious, our resources are here to guide you.
Ready to explore more comparisons and dive deeper into complex topics? Visit COMPARE.EDU.VN today!
For any inquiries, please contact us at:
Address: 333 Comparison Plaza, Choice City, CA 90210, United States
WhatsApp: +1 (626) 555-9090
Website: compare.edu.vn
