How To Compare Solubility Of Organic Compounds? Determining the solubility of organic compounds involves understanding their polarity and intermolecular interactions, a service COMPARE.EDU.VN excels at providing. By analyzing these factors, you can predict which solvents will effectively dissolve specific organic compounds. This comprehensive guide will explore the factors affecting solubility, provide practical examples, and offer tools for predicting solubility, ensuring you make informed decisions.
1. What Factors Affect The Solubility Of Organic Compounds?
The solubility of organic compounds is influenced by several key factors, including polarity, intermolecular forces, molecular size, temperature, and pressure. Understanding these factors helps predict the solubility of a compound in a given solvent.
1.1. Polarity
Polarity is a primary determinant of solubility. The golden rule, “like dissolves like,” means that polar compounds dissolve well in polar solvents, while nonpolar compounds dissolve well in nonpolar solvents.
- Polar Compounds: These compounds have an uneven distribution of electron density, leading to partial positive and negative charges. Examples include water, alcohols, and ketones.
- Nonpolar Compounds: These compounds have an even distribution of electron density and lack significant partial charges. Examples include hexane, toluene, and diethyl ether.
- Miscibility: Liquids that are completely soluble in each other are said to be miscible. For instance, ethanol and water are miscible due to their similar polarities and ability to form hydrogen bonds.
1.2. Intermolecular Forces
Intermolecular forces (IMFs) are attractive or repulsive forces between molecules. The strength and type of IMFs significantly affect solubility.
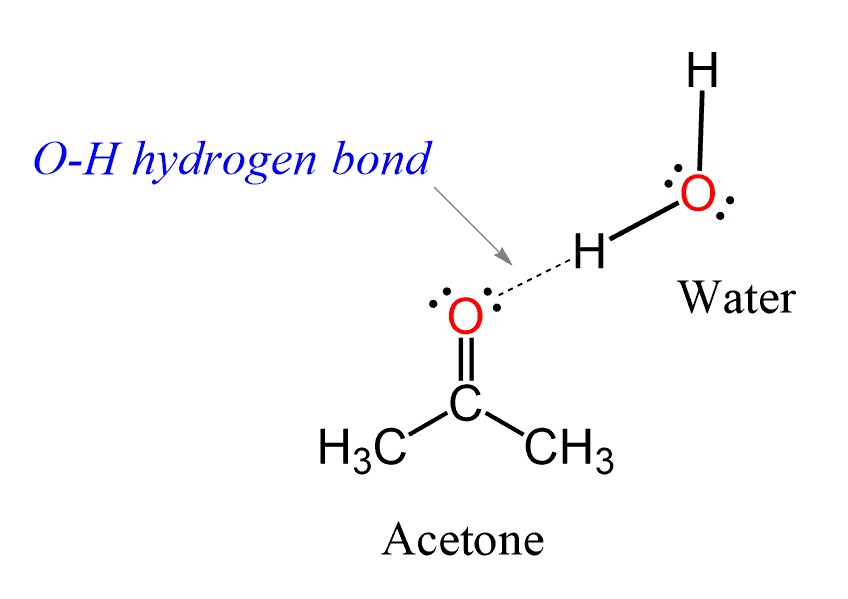
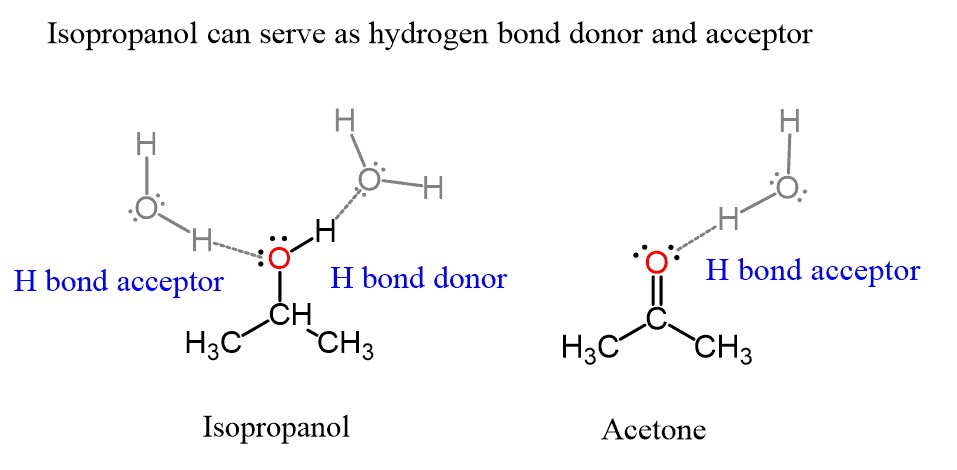
- Hydrogen Bonding: This is a strong IMF that occurs between molecules containing hydrogen bonded to highly electronegative atoms such as oxygen, nitrogen, or fluorine. Compounds that can form hydrogen bonds are highly soluble in polar solvents like water.
- Dipole-Dipole Interactions: These occur between polar molecules. The positive end of one molecule attracts the negative end of another. Polar solvents like acetone or ethyl acetate can dissolve other polar compounds through dipole-dipole interactions.
- London Dispersion Forces: These are weak, temporary attractive forces that occur between all molecules, including nonpolar ones. Nonpolar solvents like hexane rely on London dispersion forces to dissolve other nonpolar compounds.
1.3. Molecular Size
Molecular size affects solubility. Larger molecules generally have lower solubility due to increased London dispersion forces that make it more difficult for solvent molecules to surround and solvate the solute molecules.
- Example: Methanol (CH3OH) is more soluble in water than butanol (C4H9OH) because methanol is smaller and has a greater proportion of polar OH groups relative to its nonpolar carbon chain.
1.4. Temperature
Temperature typically increases the solubility of solid solutes in liquid solvents. Higher temperatures provide more kinetic energy, which helps break the intermolecular forces holding the solute together.
- Example: Sugar dissolves more readily in hot water than in cold water due to the increased kinetic energy at higher temperatures.
1.5. Pressure
Pressure has a significant effect on the solubility of gases in liquids. According to Henry’s Law, the solubility of a gas in a liquid is directly proportional to the pressure of the gas above the liquid.
- Example: Carbon dioxide is dissolved in carbonated beverages under pressure. When the pressure is released, the solubility of CO2 decreases, and it escapes as bubbles.
2. How To Predict Solubility Based On Molecular Structure?
Predicting the solubility of organic compounds based on their molecular structure involves considering the balance between polar and nonpolar regions within the molecule. Here’s how to approach this prediction:
2.1. Identify Polar and Nonpolar Groups
Begin by identifying the polar and nonpolar functional groups present in the molecule. Polar groups such as -OH, -NH2, -COOH, and -C=O increase solubility in polar solvents, while nonpolar groups such as alkyl and aryl groups decrease it.
2.2. Assess the Carbon-to-Polar Group Ratio
The ratio of carbon atoms to polar groups is crucial. Generally, the more carbon atoms relative to polar groups, the lower the solubility in polar solvents. A good rule of thumb is that for every polar group (e.g., -OH, -NH2), a compound can typically dissolve in water if it has no more than 3-5 carbon atoms.
2.3. Evaluate Intermolecular Forces
Consider the types of intermolecular forces that the solute can form with the solvent. Hydrogen bonding is particularly important for solubility in water and other protic solvents. Dipole-dipole interactions are significant for polar aprotic solvents. London dispersion forces are the primary interactions in nonpolar solvents.
2.4. Consider Molecular Shape and Size
Molecular shape and size can influence solubility. Compact, symmetrical molecules tend to pack more efficiently in a solid state, which can decrease their solubility. Larger molecules have greater surface area for London dispersion forces, potentially increasing solubility in nonpolar solvents but decreasing it in polar solvents.
2.5. Apply the “Like Dissolves Like” Principle
Match the solute with a solvent that has similar polarity and intermolecular forces. Polar solutes are more soluble in polar solvents, and nonpolar solutes are more soluble in nonpolar solvents.
2.6. Example Predictions
- Glucose (C6H12O6): Contains multiple -OH groups, making it highly soluble in water due to extensive hydrogen bonding.
- Cholesterol (C27H46O): Contains only one -OH group and a large nonpolar carbon skeleton, making it nearly insoluble in water but soluble in nonpolar solvents like hexane.
- Acetone (CH3COCH3): Polar due to the carbonyl group and has a small carbon chain, making it miscible with water and useful as a polar aprotic solvent.
- Diethyl Ether (C4H10O): Contains an oxygen atom but has a relatively large nonpolar region, making it a good solvent for nonpolar and moderately polar compounds.
3. What Are The Key Properties Of Polar Solvents And Nonpolar Solvents?
Polar and nonpolar solvents have distinct properties that dictate their ability to dissolve different types of compounds. Understanding these properties is essential for selecting the appropriate solvent for a given solute.
3.1. Polar Solvents
Polar solvents have a high dielectric constant, which measures their ability to reduce the electrostatic attraction between ions. They possess dipole moments due to uneven electron distribution and can form hydrogen bonds.
- Common Polar Solvents:
- Water (H2O): Highly polar and capable of extensive hydrogen bonding. It dissolves ionic compounds, polar molecules, and compounds that can form hydrogen bonds.
- Alcohols (e.g., Methanol, Ethanol): Polar due to the -OH group, which can form hydrogen bonds. They dissolve a variety of polar and ionic compounds.
- Acetone (CH3COCH3): Polar aprotic solvent with a high dielectric constant. It dissolves many polar organic compounds but cannot donate hydrogen bonds.
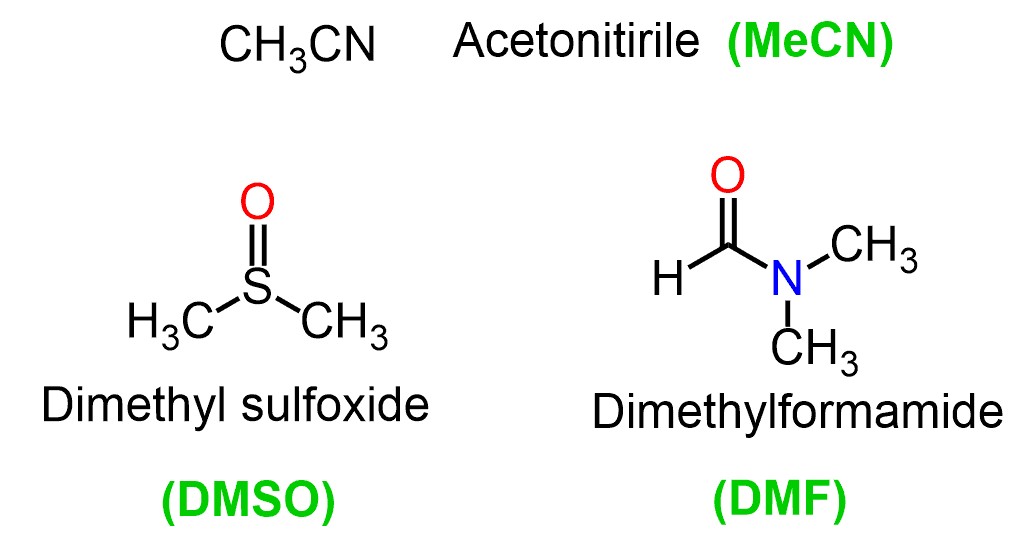
- Acetonitrile (CH3CN): Polar aprotic solvent with a high dielectric constant, commonly used in chromatography and organic synthesis.
- Dimethyl Sulfoxide (DMSO): Highly polar aprotic solvent that can dissolve a wide range of compounds, including polar and nonpolar substances.
- Dimethylformamide (DMF): Polar aprotic solvent with a high boiling point, used for dissolving polar compounds and facilitating chemical reactions.
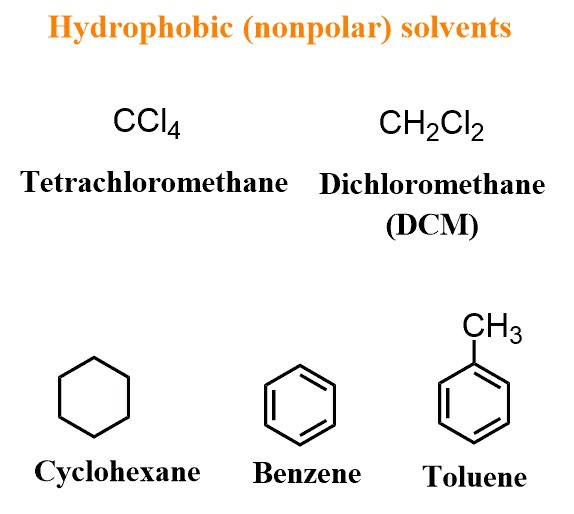
3.2. Nonpolar Solvents
Nonpolar solvents have a low dielectric constant and lack significant dipole moments. They primarily interact through London dispersion forces and are suitable for dissolving nonpolar compounds.
- Common Nonpolar Solvents:
- Hexane (C6H14): Aliphatic hydrocarbon that dissolves nonpolar compounds like oils, fats, and waxes.
- Toluene (C7H8): Aromatic hydrocarbon that dissolves nonpolar compounds. It is less volatile than hexane and often used in paints and coatings.
- Diethyl Ether (C4H10O): Nonpolar due to the dominance of the alkyl groups. It is used to dissolve nonpolar organic compounds and extract them from aqueous solutions.
- Cyclohexane (C6H12): Cyclic aliphatic hydrocarbon that dissolves nonpolar compounds and is used in various industrial processes.
- Dichloromethane (DCM or CH2Cl2): Moderately polar solvent used for extracting organic compounds and as a reaction solvent.
- Carbon Tetrachloride (CCl4): Historically used as a nonpolar solvent but now restricted due to its toxicity.
3.3. Properties Comparison Table
| Property | Polar Solvents | Nonpolar Solvents |
|---|---|---|
| Dielectric Constant | High | Low |
| Dipole Moment | Significant | Negligible |
| Intermolecular Forces | Hydrogen bonding, dipole-dipole, London dispersion | London dispersion |
| Solubility | Dissolves polar and ionic compounds | Dissolves nonpolar compounds |
| Examples | Water, alcohols, acetone, acetonitrile, DMSO, DMF | Hexane, toluene, diethyl ether, cyclohexane, DCM, CCl4 |
4. How Does Hydrogen Bonding Impact Solubility?
Hydrogen bonding significantly enhances the solubility of organic compounds in polar solvents, especially water. Compounds capable of forming hydrogen bonds with water molecules are more likely to dissolve.
4.1. The Nature of Hydrogen Bonds
A hydrogen bond is an attractive force between a hydrogen atom bonded to an electronegative atom (such as oxygen, nitrogen, or fluorine) and another electronegative atom in a different molecule or part of the same molecule.
4.2. Impact on Solubility in Water
- Alcohols: Alcohols such as methanol (CH3OH) and ethanol (C2H5OH) are highly soluble in water because they can both donate and accept hydrogen bonds. The -OH group forms strong hydrogen bonds with water molecules, facilitating dissolution.
- Amines: Amines (R-NH2) can also form hydrogen bonds with water, enhancing their solubility. Primary and secondary amines are more soluble than tertiary amines because they have more hydrogen atoms available for bonding.
- Carboxylic Acids: Carboxylic acids (R-COOH) can form hydrogen bonds through both the -OH and C=O groups, making them moderately soluble in water, especially if the carbon chain is short.
- Sugars: Sugars like glucose and sucrose have multiple -OH groups, allowing them to form numerous hydrogen bonds with water, resulting in high solubility.
4.3. Limitations of Hydrogen Bonding
The effect of hydrogen bonding on solubility diminishes as the size of the nonpolar (hydrocarbon) portion of the molecule increases. For example, butanol (C4H9OH) is less soluble in water than ethanol because the larger nonpolar butyl group reduces the overall polarity and hydrogen bonding capability of the molecule.
4.4. Examples
- Glycerol (C3H8O3): With three -OH groups, glycerol is highly soluble in water due to its extensive hydrogen bonding network.
- Phenol (C6H5OH): Phenol has a single -OH group attached to a benzene ring. Its solubility in water is lower than that of aliphatic alcohols due to the larger nonpolar aromatic ring.
- Acetic Acid (CH3COOH): Acetic acid is soluble in water because it can form hydrogen bonds through both the -OH and C=O groups.
4.5. Hydrogen Bonding in Other Solvents
Hydrogen bonding also affects solubility in other polar solvents, such as alcohols and amides. Compounds that can form hydrogen bonds are generally more soluble in these solvents than those that cannot.
5. How Does Molecular Size Affect Solubility?
Molecular size significantly influences the solubility of organic compounds. Generally, as the size of a molecule increases, its solubility in water decreases, while its solubility in nonpolar solvents may increase. This effect is primarily due to the interplay between polar and nonpolar regions within the molecule and the strength of intermolecular forces.
5.1. Solubility in Water
- Small Molecules: Small, polar molecules like methanol (CH3OH) and ethanol (C2H5OH) are highly soluble in water. Their small size allows water molecules to effectively solvate them, and their polar groups (e.g., -OH) can form hydrogen bonds with water.
- Large Molecules: As the size of the molecule increases, the nonpolar (hydrocarbon) portion becomes more dominant. This reduces the overall polarity of the molecule and its ability to form hydrogen bonds with water. For example, butanol (C4H9OH) is less soluble in water than ethanol, and octanol (C8H17OH) is practically insoluble.
- Polyfunctional Molecules: Large molecules with multiple polar functional groups may still be soluble in water if the polar groups can compensate for the nonpolar region. For example, sugars like glucose (C6H12O6) have multiple -OH groups that enable them to form extensive hydrogen bonds with water, despite their relatively large size.
5.2. Solubility in Nonpolar Solvents
- Small Molecules: Small, nonpolar molecules like methane (CH4) and ethane (C2H6) are soluble in nonpolar solvents like hexane and toluene. The primary intermolecular forces between these molecules are London dispersion forces, which are effective in nonpolar environments.
- Large Molecules: As the size of the molecule increases, the strength of the London dispersion forces also increases. This can enhance solubility in nonpolar solvents. For example, large alkanes like octadecane (C18H38) are more soluble in hexane than smaller alkanes.
- Branched Molecules: Branched molecules tend to be more soluble than linear molecules in both polar and nonpolar solvents. Branching reduces the surface area of the molecule, which decreases the strength of intermolecular forces and makes it easier for solvent molecules to solvate the solute.
5.3. Examples
- Methanol (CH3OH) vs. Octanol (C8H17OH): Methanol is highly soluble in water, while octanol is practically insoluble. The large nonpolar octyl group in octanol diminishes its ability to form hydrogen bonds with water.
- Benzene (C6H6) vs. Naphthalene (C10H8): Both benzene and naphthalene are nonpolar aromatic hydrocarbons. Naphthalene, being larger, is more soluble in nonpolar solvents like hexane than benzene due to its increased London dispersion forces.
- Glucose (C6H12O6) vs. Cellulose (C6H10O5)n: Glucose is soluble in water due to its multiple -OH groups. Cellulose, a polymer of glucose, is insoluble because its large size and crystalline structure prevent water molecules from effectively solvating it.
5.4. General Trends
- As molecular size increases, solubility in water generally decreases.
- As molecular size increases, solubility in nonpolar solvents generally increases.
- Branching can enhance solubility in both polar and nonpolar solvents.
- The balance between polar and nonpolar regions within the molecule is crucial in determining its overall solubility.
6. How Does Temperature Affect Solubility?
Temperature has a significant effect on the solubility of organic compounds, though the nature of this effect depends on whether the dissolution process is endothermic or exothermic. In most cases, increasing the temperature increases the solubility of solid solutes in liquid solvents.
6.1. Endothermic Dissolution
- Definition: An endothermic dissolution process is one that absorbs heat from the surroundings. In this case, the energy required to break the intermolecular forces within the solute and solvent is greater than the energy released when new interactions form between the solute and solvent.
- Effect of Temperature: According to Le Chatelier’s principle, if heat is added to an endothermic process, the equilibrium will shift to favor the dissolution of the solute. Thus, increasing the temperature generally increases the solubility of solids in liquid solvents when the dissolution process is endothermic.
- Examples:
- The solubility of most ionic compounds in water increases with temperature. For instance, the solubility of potassium nitrate (KNO3) in water increases significantly as the temperature rises.
- Many organic compounds also exhibit increased solubility with temperature when dissolving in polar or nonpolar solvents.
6.2. Exothermic Dissolution
- Definition: An exothermic dissolution process is one that releases heat into the surroundings. In this case, the energy released when new interactions form between the solute and solvent is greater than the energy required to break the intermolecular forces within the solute and solvent.
- Effect of Temperature: According to Le Chatelier’s principle, if heat is added to an exothermic process, the equilibrium will shift to favor the reactants (i.e., the undissolved solute). Thus, increasing the temperature generally decreases the solubility of gases in liquids and, in some cases, the solubility of certain solids.
- Examples:
- The solubility of gases in liquids typically decreases with increasing temperature. For instance, the solubility of oxygen (O2) and carbon dioxide (CO2) in water decreases as the temperature rises. This is why warm soda goes flat more quickly than cold soda.
- Some solid organic compounds may exhibit decreased solubility with increasing temperature if the dissolution process is highly exothermic.
6.3. Temperature and Solubility in Nonpolar Solvents
In nonpolar solvents, the effect of temperature on solubility is often less pronounced than in polar solvents. The dissolution process usually involves breaking and forming London dispersion forces, which are relatively weak. As a result, the heat of dissolution is typically small, and the temperature dependence of solubility is less significant.
6.4. Practical Implications
- Recrystallization: Temperature dependence of solubility is often used in recrystallization, a common technique for purifying solid organic compounds. The compound is dissolved in a hot solvent, and then the solution is cooled, causing the compound to crystallize out as the solubility decreases.
- Extraction: Temperature can also affect the efficiency of liquid-liquid extraction. In some cases, heating the mixture can increase the solubility of the desired compound in the extracting solvent, improving the yield.
7. What Are Some Common Solvents Used In Organic Chemistry?
Organic chemistry relies on a variety of solvents, each with specific properties that make them suitable for different applications. These solvents can be broadly categorized as polar protic, polar aprotic, and nonpolar.
7.1. Polar Protic Solvents
Polar protic solvents are capable of donating hydrogen bonds and have high dielectric constants. They are excellent for dissolving ionic compounds and polar molecules.
- Water (H2O): The most common polar protic solvent, ideal for dissolving ionic compounds, polar molecules, and compounds that can form hydrogen bonds.
- Methanol (CH3OH): A versatile solvent for polar compounds, with a lower boiling point than water, making it easier to remove.
- Ethanol (C2H5OH): Similar to methanol but slightly less polar. It is commonly used in recrystallization and extraction processes.
- Acetic Acid (CH3COOH): Used as a solvent and reagent, particularly in reactions involving carboxylic acids.
7.2. Polar Aprotic Solvents
Polar aprotic solvents have high dielectric constants but cannot donate hydrogen bonds. They are useful for dissolving polar compounds and facilitating reactions involving strong bases and nucleophiles.
- Acetone (CH3COCH3): A good solvent for polar organic compounds, commonly used in cleaning and extraction processes.
- Acetonitrile (CH3CN): A versatile solvent for chromatography and organic synthesis, with a relatively low boiling point.
- Dimethyl Sulfoxide (DMSO): A highly polar solvent that can dissolve a wide range of compounds, including polar and nonpolar substances. It is often used in reactions involving organometallic reagents.
- Dimethylformamide (DMF): A high-boiling-point solvent used for dissolving polar compounds and facilitating chemical reactions.
- Ethyl Acetate (CH3COOC2H5): A moderately polar solvent often used in extraction and chromatography due to its low toxicity and ease of removal.
7.3. Nonpolar Solvents
Nonpolar solvents have low dielectric constants and are primarily used to dissolve nonpolar compounds.
- Hexane (C6H14): A common aliphatic hydrocarbon solvent for nonpolar compounds like oils, fats, and waxes.
- Toluene (C7H8): An aromatic hydrocarbon solvent used for dissolving nonpolar compounds and as a component in paints and coatings.
- Diethyl Ether (C4H10O): A volatile solvent used for extracting organic compounds from aqueous solutions and as a reaction solvent.
- Cyclohexane (C6H12): A cyclic aliphatic hydrocarbon solvent for nonpolar compounds, used in various industrial processes.
- Dichloromethane (DCM or CH2Cl2): A moderately polar solvent used for extracting organic compounds and as a reaction solvent.
7.4. Solvent Selection Considerations
- Solubility of Reactants and Products: Choose a solvent that dissolves the reactants and, ideally, the products of the reaction.
- Reactivity: Ensure that the solvent does not react with the reactants or reagents.
- Boiling Point: Select a solvent with a suitable boiling point for the reaction temperature and ease of removal.
- Toxicity: Consider the toxicity of the solvent and choose a less toxic alternative whenever possible.
- Cost: Balance the performance of the solvent with its cost.
8. What Role Does Solvent Polarity Play In Chemical Reactions?
Solvent polarity plays a crucial role in chemical reactions, affecting reaction rates, mechanisms, and selectivity. The choice of solvent can significantly influence the stability of reactants, transition states, and products, thereby determining the outcome of a reaction.
8.1. SN1 Reactions
SN1 reactions (unimolecular nucleophilic substitution) involve the formation of a carbocation intermediate. Polar protic solvents favor SN1 reactions because they stabilize the carbocation intermediate through solvation. The polar solvent molecules surround the carbocation, reducing its energy and making its formation more favorable.
- Examples: Water, alcohols (e.g., ethanol, methanol), and carboxylic acids.
8.2. SN2 Reactions
SN2 reactions (bimolecular nucleophilic substitution) involve a concerted mechanism where the nucleophile attacks the substrate at the same time as the leaving group departs. Polar aprotic solvents favor SN2 reactions because they do not solvate the nucleophile as strongly as polar protic solvents do. This makes the nucleophile more reactive and enhances the rate of the SN2 reaction.
- Examples: Acetone, acetonitrile, dimethyl sulfoxide (DMSO), and dimethylformamide (DMF).
8.3. E1 Reactions
E1 reactions (unimolecular elimination) are similar to SN1 reactions in that they involve the formation of a carbocation intermediate. Polar protic solvents also favor E1 reactions for the same reason they favor SN1 reactions: they stabilize the carbocation intermediate.
8.4. E2 Reactions
E2 reactions (bimolecular elimination) involve a concerted mechanism where the base removes a proton from the substrate at the same time as the leaving group departs. Polar aprotic solvents can also favor E2 reactions, particularly when strong, bulky bases are used. The aprotic solvent does not solvate the base, making it more reactive and promoting the elimination reaction.
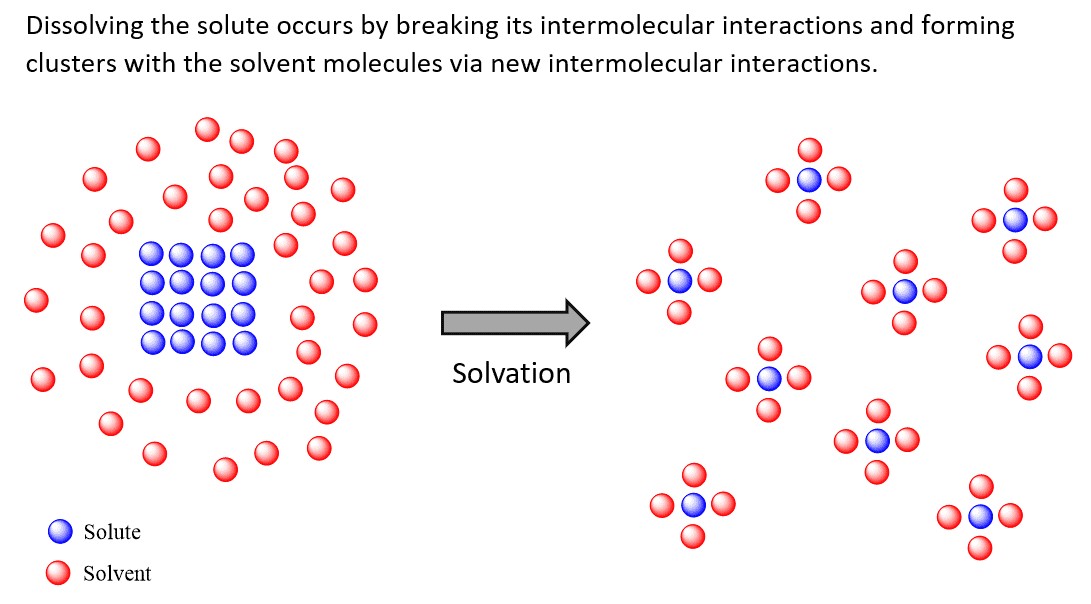
8.5. Solvation Effects
Solvents can affect the stability of reactants and products through solvation. Solvation is the process by which solvent molecules surround and interact with solute molecules or ions. Polar solvents tend to solvate polar and ionic species, while nonpolar solvents tend to solvate nonpolar species.
- Stabilization of Charged Species: Polar solvents stabilize charged species by distributing the charge over a larger volume. This reduces the electrostatic interactions and lowers the energy of the charged species.
- Destabilization of Transition States: In some cases, solvents can destabilize transition states, leading to slower reaction rates. For example, nonpolar solvents can destabilize charged transition states, making the reaction less favorable.
8.6. Dielectric Constant
The dielectric constant of a solvent is a measure of its ability to reduce the electrostatic interactions between ions. Solvents with high dielectric constants are better at stabilizing charged species and promoting reactions that involve charged intermediates or transition states.
8.7. Specific Examples
- Grignard Reactions: Grignard reagents (RMgX) are strong nucleophiles that react with carbonyl compounds to form alcohols. These reactions are typically carried out in anhydrous diethyl ether or tetrahydrofuran (THF) because water and alcohols will react with the Grignard reagent and destroy it.
- Diels-Alder Reactions: Diels-Alder reactions are cycloaddition reactions between a diene and a dienophile to form a cyclic product. These reactions can be carried out in a variety of solvents, but nonpolar solvents like toluene and hexane are often preferred because they do not interfere with the reaction and can sometimes increase the reaction rate through favorable interactions with the transition state.
9. What Is The Relationship Between Solubility And Intermolecular Forces?
The relationship between solubility and intermolecular forces is fundamental to understanding why certain compounds dissolve in specific solvents. Solubility is the ability of a substance (solute) to dissolve in a solvent, and it is primarily determined by the strength and nature of the intermolecular forces (IMFs) between the solute and solvent molecules. The principle that “like dissolves like” encapsulates this relationship.
9.1. Types of Intermolecular Forces
- Hydrogen Bonding: A strong IMF that occurs between molecules containing hydrogen bonded to highly electronegative atoms such as oxygen, nitrogen, or fluorine.
- Dipole-Dipole Interactions: Attractive forces between polar molecules. These forces occur when the positive end of one molecule is attracted to the negative end of another.
- London Dispersion Forces (LDF): Weak, temporary attractive forces that occur between all molecules, including nonpolar ones. These forces arise from temporary fluctuations in electron distribution.
9.2. Solubility in Polar Solvents
Polar solvents, such as water and alcohols, are effective at dissolving solutes that can form strong IMFs with them.
- Hydrogen Bonding: Solutes that can form hydrogen bonds with polar solvents exhibit high solubility. For example, alcohols like ethanol (C2H5OH) and sugars like glucose (C6H12O6) are highly soluble in water due to their ability to form hydrogen bonds.
- Dipole-Dipole Interactions: Polar solutes with dipole moments can dissolve in polar solvents through dipole-dipole interactions. For instance, acetone (CH3COCH3) and acetonitrile (CH3CN) are soluble in water because their polar C=O and C≡N groups can interact with the dipole of water molecules.
- Ionic Compounds: Ionic compounds, such as sodium chloride (NaCl) and potassium iodide (KI), dissolve in polar solvents because the ions are strongly solvated by the solvent molecules. The positive ions (cations) are surrounded by the negative ends of the solvent molecules, and the negative ions (anions) are surrounded by the positive ends of the solvent molecules.
9.3. Solubility in Nonpolar Solvents
Nonpolar solvents, such as hexane and toluene, are effective at dissolving solutes that can form weak IMFs with them.
- London Dispersion Forces: Nonpolar solutes dissolve in nonpolar solvents because the primary IMFs between the molecules are London dispersion forces. For example, alkanes like hexane (C6H14) and aromatic hydrocarbons like benzene (C6H6) are soluble in nonpolar solvents.
- Induced Dipole Interactions: Nonpolar solutes can induce temporary dipoles in nonpolar solvents, leading to weak attractive forces that promote solubility.
9.4. Factors Affecting the Strength of IMFs
- Polarizability: The polarizability of a molecule is a measure of its ability to form temporary dipoles. Larger molecules with more electrons are generally more polarizable and exhibit stronger London dispersion forces.
- Molecular Shape: The shape of a molecule can also affect the strength of IMFs. Linear molecules can pack more closely together than branched molecules, leading to stronger IMFs.
- Temperature: Temperature affects the kinetic energy of molecules, which can influence the strength of IMFs. Higher temperatures can disrupt IMFs and decrease solubility in some cases.
9.5. Examples Illustrating the Relationship
- Water vs. Hexane: Water is a polar solvent that dissolves polar solutes like ethanol and glucose, while hexane is a nonpolar solvent that dissolves nonpolar solutes like oils and waxes.
- Ethanol vs. Octanol: Ethanol (C2H5OH) is highly soluble in water due to its small size and ability to form hydrogen bonds, while octanol (C8H17OH) is practically insoluble because its large nonpolar alkyl chain reduces its ability to form hydrogen bonds.
- Sodium Chloride vs. Benzene: Sodium chloride (NaCl) is an ionic compound that dissolves in water due to ion-dipole interactions, while benzene (C6H6) is a nonpolar compound that dissolves in nonpolar solvents like hexane due to London dispersion forces.
10. What Are Some Techniques For Enhancing Solubility?
Enhancing the solubility of a compound involves altering the conditions or properties of the solute or solvent to promote greater dissolution. Several techniques can be employed, depending on the nature of the solute and solvent.
10.1. Temperature Control
- Heating: Increasing the temperature generally increases the solubility of solid solutes in liquid solvents, especially for endothermic dissolution processes. Heating provides more kinetic energy to break the intermolecular forces holding the solute together.
- Cooling: Cooling can be used to induce crystallization of a solute from a supersaturated solution. This technique is often used in recrystallization to purify solid compounds.
10.2. Solvent Selection
- Choosing a More Compatible Solvent: Selecting a solvent that is more chemically similar to the solute can significantly enhance solubility. This is based on the principle “like dissolves like.” For instance, using a polar solvent for a polar solute or a nonpolar solvent for a nonpolar solute.
- Solvent Mixtures: Using a mixture of solvents can sometimes enhance solubility. For example, a mixture of water and ethanol can dissolve compounds that are not soluble in either solvent alone.
10.3. Salt Formation
- Acidic or Basic Solutes: Converting an acidic or basic solute into its salt form can dramatically increase its solubility in water. For example, a carboxylic acid can be converted into its sodium salt by reacting it with sodium hydroxide. The resulting salt is often much more soluble in water than the original acid. Similarly, an amine can be converted into its hydrochloride salt by reacting it with hydrochloric acid.
10.4. Complexation
- Complexing Agents: Adding a complexing agent can increase the solubility of a solute by forming a soluble complex with it. For example, adding cyclodextrins can enhance the solubility of hydrophobic drugs by encapsulating them in their hydrophobic cavities.
10.5. Cosolvency
- Adding a Cosolvent: A cosolvent is a substance that increases the solubility of a solute in a given solvent. Cosolvents work by altering the properties of the solvent to make it more compatible with the solute. For example, adding glycerol or propylene glycol to water can increase the solubility of poorly soluble drugs.
10.6. Particle Size Reduction
- Micronization: Reducing the particle size of a solid solute can increase its surface area, which enhances its dissolution rate. Micronization involves grinding the solute into very fine particles.
- Nanoparticles: Formulating a solute as nanoparticles can further increase its surface area and enhance its solubility and dissolution rate.
10.7. Surfactants
- Adding Surfactants: Surfactants are amphiphilic molecules that have both hydrophilic (water-loving) and hydrophobic (water-hating) regions. They can increase the solubility of hydrophobic compounds in water by forming micelles, in which the hydrophobic regions of the surfactants surround the solute molecules, while the hydrophilic regions interact with the water.
10.8. pH Adjustment
- Weak Acids or Bases: Adjusting the pH of the solution can enhance the solubility of weak acids or bases. For example, increasing the pH can increase the solubility of a weak acid by converting it into its ionized form.
Struggling to compare the solubility of organic compounds? Visit COMPARE.EDU.VN for comprehensive, easy-to-understand comparisons and expert insights. Make informed decisions and optimize your experiments with our detailed analyses. Contact us at 333 Comparison Plaza, Choice City, CA 90210, United States, or Whatsapp: +1 (626) 555-9090. compare.edu.vn – Where informed decisions begin.
Frequently Asked Questions (FAQs)
-
What does “like dissolves like” mean in the context of solubility?
The principle “like dissolves like” means that polar compounds dissolve best in polar solvents, while nonpolar compounds dissolve best in nonpolar solvents. This is due to the intermolecular forces between the solute and solvent molecules. -
How does hydrogen bonding affect the solubility of organic compounds in water?
Hydrogen bonding significantly increases the solubility of organic compounds in water because water molecules can form strong hydrogen bonds with compounds containing -OH, -NH, or -FH groups. -
Why does increasing the temperature usually increase the solubility of solid solutes in liquid solvents?
Increasing the temperature provides more kinetic energy, which helps break the intermolecular forces holding the solute together and allows the solvent molecules to solvate the solute more effectively. -
What are some common polar aprotic solvents used in organic chemistry?
Common polar aprotic solvents include acetone, acetonitrile, dimethyl sulfoxide (DMSO), and dimethylformamide (DMF). These solvents have high dielectric constants but cannot donate hydrogen bonds. -
How does molecular size affect the solubility of organic compounds in water?
Generally, as the size of an organic molecule increases, its solubility in water decreases because the nonpolar (hydrocarbon) portion becomes more dominant, reducing the molecule’s ability to form hydrogen bonds with water. -
What is the role of surfactants in enhancing the solubility of hydrophobic compounds in water?
Surfactants are amphiphilic molecules that can form micelles in water, with their hydrophobic regions surrounding the solute molecules and their hydrophilic regions interacting with the water, thus enhancing the solubility of hydrophobic compounds. -
How does the dielectric constant of a solvent affect its ability to dissolve ionic compounds?
Solvents with high dielectric constants are better at stabilizing charged species, making them more effective at dissolving ionic compounds by reducing the electrostatic interactions between the ions. -
What are some techniques used to enhance the solubility of poorly soluble drugs?
Techniques to enhance drug solubility include salt formation, micronization, complexation with cyclodextrins, the use of cosolvents like glycerol, and formulating the drug as nanoparticles. -
Why are Grignard reactions typically carried out in anhydrous diethyl ether or tetrahydrofuran (THF)?
Grignard reagents are strong nucleophiles that react with carbonyl compounds to form alcohols. Anhydrous diethyl ether or THF is used because water and alcohols will react with the Grignard reagent and destroy it. -
How does pH adjustment affect the solubility of weak acids and bases?
Adjusting the pH of the solution can enhance the solubility of weak acids or bases. Increasing the pH can increase the solubility of a weak acid by converting it into its ionized form, while decreasing the pH can increase the solubility of a weak base.