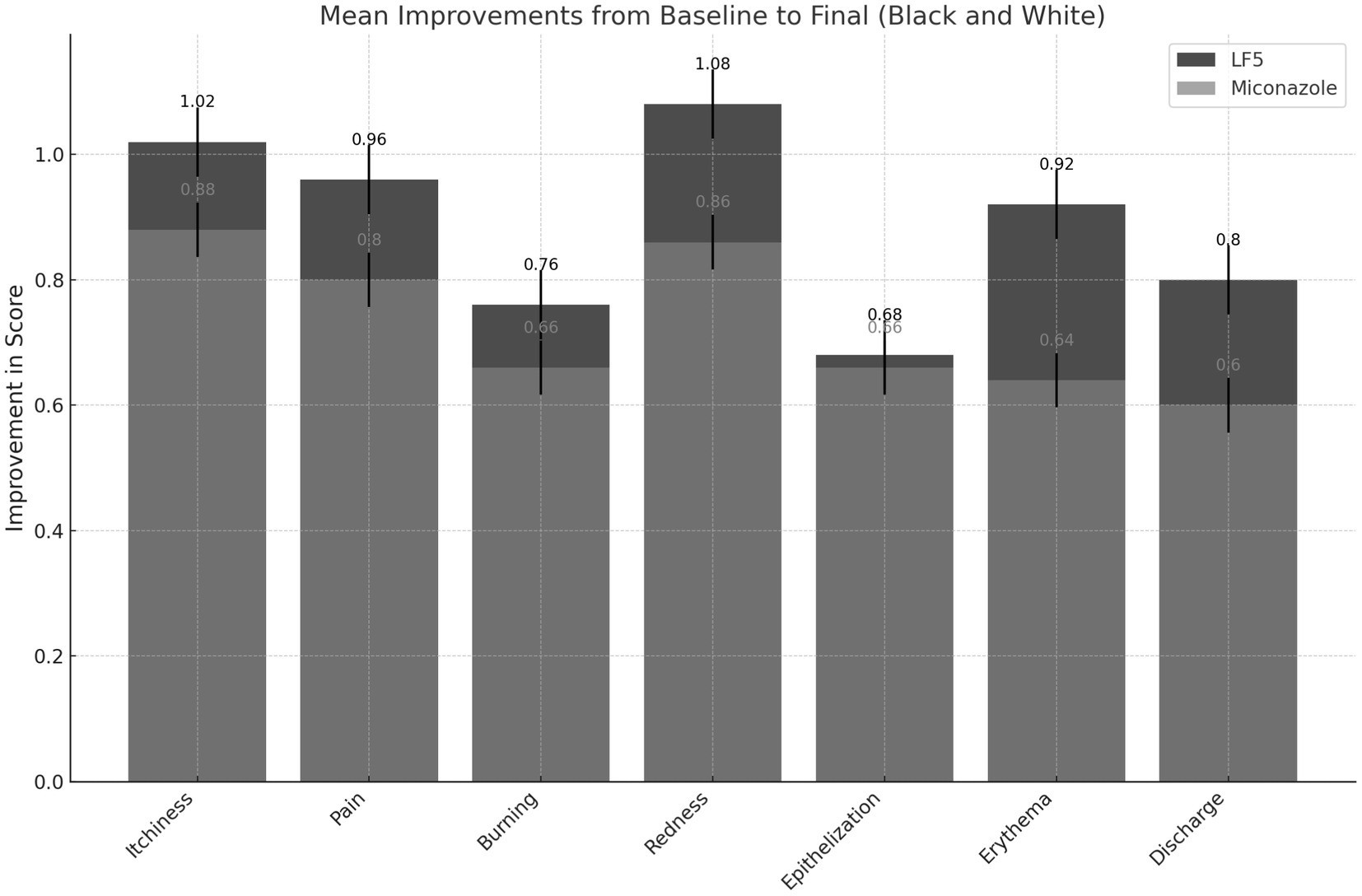
A Single-blind Randomized Comparative Study is valuable for assessing treatment efficacy, especially in conditions like vulvovaginal candidiasis (VVC). At COMPARE.EDU.VN, we provide in-depth analyses that help clarify the advantages and disadvantages of these studies, offering a comprehensive comparison. Optimize your understanding with our thorough treatment evaluations, assessment methodologies, and clinical research insights.
1. What Is A Single-Blind Randomized Comparative Study?
A single-blind randomized comparative study is a research method where participants are unaware of which treatment they are receiving, while the researchers are aware. This design aims to minimize bias by ensuring that participant expectations do not influence the results. Single-blind studies are particularly useful when comparing a new treatment against a standard treatment or a placebo.
1.1. Key Elements Of A Single-Blind Randomized Comparative Study
A single-blind randomized comparative study includes the following key elements:
- Randomization: Participants are randomly assigned to different treatment groups to ensure that each participant has an equal chance of being in any group, reducing selection bias.
- Blinding: Participants are not informed about which treatment they are receiving (either the active treatment or a placebo).
- Comparison: At least two groups are compared: one receiving the active treatment and another receiving a control (placebo or standard treatment).
- Control Group: A control group provides a baseline against which the effects of the active treatment can be measured. This can be a placebo group or a group receiving the current standard treatment.
1.2. Why Use A Single-Blind Study Design?
The primary reason for using a single-blind design is to mitigate the placebo effect, where participants may experience perceived benefits simply because they believe they are receiving treatment. By keeping participants unaware of their treatment assignment, researchers can better isolate the true effects of the intervention. This is especially important in studies involving subjective outcomes, such as pain relief or symptom improvement.
1.3. Limitations Of Single-Blind Studies
Despite their advantages, single-blind studies have limitations. Since researchers are aware of the treatment assignments, they may unintentionally introduce bias in how they interact with participants or interpret the results. This is known as observer bias or experimenter bias. In such cases, a double-blind study, where both participants and researchers are unaware of treatment assignments, may be more appropriate.
2. What Are The Advantages Of Single-Blind Randomized Comparative Studies?
Single-blind randomized comparative studies offer several advantages over other study designs, particularly in clinical research. These advantages include reducing participant bias, maintaining ethical standards, and providing a balance between feasibility and rigor.
2.1. Reduction Of Participant Bias
One of the primary advantages of single-blind studies is the reduction of participant bias. When participants are unaware of whether they are receiving an active treatment or a placebo, their expectations are less likely to influence their reported outcomes. This is particularly important in studies where subjective measures, such as pain levels or perceived well-being, are used.
2.2. Ethical Considerations
In some situations, it may be unethical or impractical to conduct a double-blind study. For instance, if the treatments being compared have obvious differences in administration (e.g., surgery versus medication), it may be impossible to blind the researchers. In such cases, a single-blind design provides a reasonable compromise that minimizes bias while adhering to ethical standards.
2.3. Feasibility
Single-blind studies can be more feasible to implement compared to double-blind studies. Blinding researchers often requires additional resources and logistical planning, which may not always be available. By allowing researchers to remain aware of treatment assignments, single-blind studies can simplify the research process without sacrificing too much in terms of bias control.
2.4. Clinical Equipoise
Single-blind studies ensure clinical equipoise, a state where there is genuine uncertainty among experts about which treatment is more beneficial. By randomizing participants to different treatments, researchers can ethically compare interventions, as there is no prior knowledge suggesting one treatment is superior.
2.5. Standardization
Single-blind studies allow for standardization of treatment protocols, ensuring that each participant receives a consistent intervention. This standardization enhances the reliability and validity of the study results. Standardized protocols help reduce variability and make it easier to compare outcomes across different treatment groups.
3. What Are The Disadvantages Of Single-Blind Randomized Comparative Studies?
Despite their advantages, single-blind randomized comparative studies also have certain disadvantages that must be considered. These include potential researcher bias, limited blinding, and the possibility of influencing the interpretation of results.
3.1. Potential For Researcher Bias
One of the main disadvantages of single-blind studies is the potential for researcher bias. Since the researchers are aware of which treatment each participant is receiving, they may unintentionally influence the results. This can occur through subtle differences in how they interact with participants, how they collect data, or how they interpret the findings.
3.2. Limited Blinding
In single-blind studies, only the participants are blinded, meaning that the researchers are aware of the treatment assignments. This limited blinding can introduce bias, especially when outcomes are subjective or require interpretation. Researchers may unconsciously look for or emphasize results that confirm their expectations or preferences.
3.3. Risk Of Performance Bias
Performance bias can occur when researchers treat participants differently based on their knowledge of treatment assignments. For example, a researcher might spend more time or offer more encouragement to participants receiving the active treatment compared to those receiving the placebo, thereby influencing the outcomes.
3.4. Assessment Bias
Assessment bias can arise when researchers evaluate outcomes differently depending on their knowledge of treatment assignments. This is particularly problematic when assessing subjective outcomes, such as symptom severity or quality of life, where the researcher’s interpretation can be influenced by their expectations.
3.5. Difficulty In Maintaining Objectivity
Maintaining objectivity can be challenging in single-blind studies because the researchers’ awareness of treatment assignments can unconsciously affect their behavior and judgment. This can lead to biased data collection, analysis, and interpretation, which can compromise the validity of the study results.
4. How Does A Single-Blind Study Compare To A Double-Blind Study?
Single-blind and double-blind studies are both used to minimize bias in research, but they differ in who is aware of the treatment assignments. A single-blind study only blinds the participants, while a double-blind study blinds both the participants and the researchers. The choice between these two designs depends on the specific goals and constraints of the research.
4.1. Blinding Of Participants
In both single-blind and double-blind studies, the participants are unaware of whether they are receiving the active treatment or a placebo. This helps to reduce participant bias and the placebo effect, where participants may experience perceived benefits simply because they believe they are receiving treatment.
4.2. Blinding Of Researchers
The key difference between single-blind and double-blind studies lies in whether the researchers are also blinded. In a single-blind study, the researchers are aware of the treatment assignments, whereas, in a double-blind study, they are not. Blinding the researchers helps to eliminate observer bias, where the researchers’ expectations or preferences can influence how they interact with participants or interpret the results.
4.3. Reduction Of Bias
Double-blind studies generally offer a higher level of bias control compared to single-blind studies. By blinding both participants and researchers, double-blind studies minimize the potential for both participant bias and observer bias, providing more objective and reliable results.
4.4. Complexity And Feasibility
Double-blind studies can be more complex and challenging to implement compared to single-blind studies. Blinding the researchers often requires additional resources and logistical planning, such as the use of a third party to prepare and administer treatments. In some cases, it may be impossible to blind the researchers, such as when the treatments being compared have obvious differences in administration.
4.5. When To Use Each Design
The choice between a single-blind and a double-blind design depends on the specific context of the research. If the primary concern is participant bias and it is not feasible to blind the researchers, a single-blind study may be appropriate. However, if observer bias is a significant concern and it is feasible to blind the researchers, a double-blind study is generally preferred.
5. What Are The Ethical Considerations In Single-Blind Studies?
Ethical considerations are paramount in single-blind studies, ensuring that research is conducted responsibly and respects the rights and well-being of participants. Key ethical principles include informed consent, minimizing risks, maintaining confidentiality, and ensuring transparency.
5.1. Informed Consent
Informed consent is a critical ethical requirement in all research involving human participants. Participants must be provided with comprehensive information about the study, including its purpose, procedures, potential risks and benefits, and their right to withdraw at any time without penalty. This information must be presented in a clear and understandable manner, allowing participants to make an informed decision about whether to participate.
5.2. Minimizing Risks
Researchers have an ethical obligation to minimize the risks to participants. This includes both physical and psychological risks. In single-blind studies, researchers must carefully assess the potential risks associated with the treatments being compared and take steps to mitigate these risks. This may involve monitoring participants closely for adverse events and providing appropriate medical care as needed.
5.3. Confidentiality
Maintaining the confidentiality of participants’ data is essential. Researchers must protect participants’ privacy by ensuring that their personal information is not disclosed to unauthorized individuals. This includes storing data securely, using anonymous coding systems, and obtaining participants’ consent before sharing any information with third parties.
5.4. Transparency
Transparency is crucial for maintaining trust and integrity in research. Researchers should be transparent about the study’s design, methods, and results. This includes disclosing any potential conflicts of interest and reporting all findings, even those that do not support the researchers’ hypotheses. Transparency also involves providing participants with access to the study results and answering any questions they may have about the research.
5.5. Institutional Review Board (IRB) Approval
Before conducting any research involving human participants, researchers must obtain approval from an Institutional Review Board (IRB). An IRB is a committee that reviews research proposals to ensure that they meet ethical standards and protect the rights and welfare of participants. The IRB will assess the study’s design, procedures, and informed consent process to ensure that they comply with ethical guidelines and regulations.
6. How Is Data Analyzed In A Single-Blind Randomized Comparative Study?
Data analysis in a single-blind randomized comparative study involves a systematic approach to compare the outcomes between the treatment and control groups. This typically includes descriptive statistics, inferential statistics, and subgroup analyses to provide a comprehensive understanding of the treatment effects.
6.1. Descriptive Statistics
Descriptive statistics are used to summarize and describe the characteristics of the study sample and the outcomes in each treatment group. This includes measures such as means, standard deviations, medians, and ranges for continuous variables, and frequencies and percentages for categorical variables. Descriptive statistics provide a clear picture of the data and help to identify any potential differences between the treatment groups.
6.2. Inferential Statistics
Inferential statistics are used to draw conclusions about the population based on the sample data. This involves using statistical tests to determine whether the observed differences between the treatment groups are statistically significant, meaning that they are unlikely to have occurred by chance. Common statistical tests used in single-blind randomized comparative studies include t-tests, ANOVA, chi-square tests, and regression analyses.
6.3. Intention-To-Treat Analysis
Intention-to-treat (ITT) analysis is a method of analyzing data in which all participants are included in the treatment group to which they were originally randomized, regardless of whether they completed the treatment or deviated from the protocol. ITT analysis helps to preserve the benefits of randomization and provides a more conservative estimate of the treatment effect.
6.4. Per-Protocol Analysis
Per-protocol (PP) analysis is another method of analyzing data in which only participants who completed the treatment and adhered to the protocol are included. PP analysis can provide a more optimistic estimate of the treatment effect, but it may also be more susceptible to bias if participants who dropped out of the study differed systematically from those who completed it.
6.5. Subgroup Analysis
Subgroup analysis involves examining the treatment effects in different subgroups of participants, such as those defined by age, sex, or disease severity. Subgroup analysis can help to identify whether the treatment is more effective in certain populations and can provide insights into the mechanisms of action of the treatment. However, subgroup analyses should be interpreted with caution, as they may be prone to false-positive findings due to multiple comparisons.
7. What Are Some Examples Of Single-Blind Studies In Medical Research?
Single-blind studies are commonly used in medical research to evaluate the effectiveness of new treatments and interventions. Several examples illustrate the application of single-blind studies in various medical fields.
7.1. Pain Management
Single-blind studies are frequently used to assess the efficacy of pain medications. In these studies, patients are randomly assigned to receive either the active pain medication or a placebo. The patients are not informed about which treatment they are receiving, but the researchers are aware. This design helps to reduce patient bias and the placebo effect, allowing for a more accurate assessment of the medication’s effectiveness.
7.2. Mental Health
Single-blind studies are also used in mental health research to evaluate the effectiveness of new therapies and interventions. For example, a single-blind study might compare the effects of a new antidepressant medication to those of a placebo. Patients are not told which treatment they are receiving, but the researchers are aware. This design helps to minimize patient expectations and biases, providing a more objective assessment of the treatment’s efficacy.
7.3. Rehabilitation
In rehabilitation research, single-blind studies can be used to evaluate the effectiveness of new rehabilitation techniques and devices. For instance, a single-blind study might compare the effects of a new physical therapy intervention to those of a standard therapy. Patients are not informed about which treatment they are receiving, but the therapists are aware. This design helps to reduce patient bias and allows for a more accurate assessment of the intervention’s effectiveness.
7.4. Surgical Interventions
In some cases, it may be impossible to conduct a double-blind study of a surgical intervention because the surgeon needs to know which procedure is being performed. In these situations, a single-blind study may be used, where the patients are not informed about which surgical procedure they are receiving, but the surgeons are aware. This design helps to reduce patient bias and allows for a more objective assessment of the intervention’s effectiveness.
7.5. Vaccine Trials
Single-blind studies can be used in vaccine trials, particularly in the early phases of research. In these studies, participants are randomly assigned to receive either the vaccine or a placebo. Participants are not informed about which treatment they are receiving, but the researchers are aware. This design helps to reduce participant bias and the placebo effect, allowing for a more accurate assessment of the vaccine’s safety and immunogenicity.
8. What Is The Role Of Randomization In A Single-Blind Study?
Randomization is a fundamental component of a single-blind study, ensuring that participants have an equal chance of being assigned to either the treatment group or the control group. This process minimizes selection bias and enhances the validity of the study results.
8.1. Minimizing Selection Bias
Randomization helps to minimize selection bias, which occurs when participants are not assigned to treatment groups in a random manner. If researchers were allowed to choose which participants received the active treatment and which received the placebo, they might consciously or unconsciously assign certain types of participants to one group or the other, thereby skewing the results.
8.2. Creating Comparable Groups
Randomization helps to create comparable groups at baseline. By randomly assigning participants to treatment groups, researchers can ensure that the groups are similar in terms of important characteristics, such as age, sex, disease severity, and other relevant factors. This makes it easier to attribute any observed differences in outcomes to the treatment rather than to pre-existing differences between the groups.
8.3. Enhancing Internal Validity
Randomization enhances the internal validity of the study, which refers to the extent to which the study results accurately reflect the true effects of the treatment. By minimizing selection bias and creating comparable groups, randomization increases the likelihood that any observed differences in outcomes are due to the treatment rather than to confounding variables.
8.4. Methods Of Randomization
Several methods can be used to randomize participants to treatment groups in a single-blind study. These include simple randomization, block randomization, and stratified randomization. Simple randomization involves using a random number generator to assign participants to treatment groups. Block randomization involves dividing participants into blocks and then randomly assigning them to treatment groups within each block. Stratified randomization involves dividing participants into strata based on important characteristics and then randomly assigning them to treatment groups within each stratum.
8.5. Importance Of Concealment
Concealment of allocation is another important aspect of randomization. This refers to the process of ensuring that researchers are not aware of which treatment a participant will receive until after the participant has been enrolled in the study. Concealment of allocation helps to prevent selection bias by ensuring that researchers cannot consciously or unconsciously influence which participants are assigned to which treatment group.
9. How Do You Interpret The Results Of A Single-Blind Study?
Interpreting the results of a single-blind study requires careful consideration of several factors, including the statistical significance of the findings, the clinical significance of the findings, and the potential for bias.
9.1. Statistical Significance
Statistical significance refers to the likelihood that the observed differences between the treatment groups are due to chance. In a single-blind study, the statistical significance of the findings is typically assessed using statistical tests, such as t-tests, ANOVA, chi-square tests, and regression analyses. A statistically significant finding is one that is unlikely to have occurred by chance, typically defined as a p-value of less than 0.05.
9.2. Clinical Significance
Clinical significance refers to the practical importance of the findings. Even if a finding is statistically significant, it may not be clinically significant if the observed effect size is small or if the treatment is associated with significant side effects. In a single-blind study, the clinical significance of the findings should be assessed by considering the magnitude of the treatment effect, the potential benefits to patients, and the potential risks and costs associated with the treatment.
9.3. Potential For Bias
In a single-blind study, it is important to consider the potential for bias, particularly observer bias. Since the researchers are aware of which treatment each participant is receiving, they may unintentionally influence the results. This can occur through subtle differences in how they interact with participants, how they collect data, or how they interpret the findings. Researchers should take steps to minimize the potential for bias, such as using standardized protocols, blinding outcome assessors, and conducting sensitivity analyses.
9.4. Generalizability
Generalizability refers to the extent to which the study results can be applied to other populations and settings. In a single-blind study, it is important to consider the characteristics of the study sample and the setting in which the study was conducted. The results may not be generalizable to other populations or settings if the study sample was not representative or if the study was conducted in a highly specialized environment.
9.5. Consistency With Other Evidence
The results of a single-blind study should be interpreted in the context of other available evidence. If the findings are consistent with those of other studies, this increases confidence in the validity of the results. However, if the findings are inconsistent with those of other studies, this may raise concerns about the validity of the results and may warrant further investigation.
10. What Are The Recent Advances In Single-Blind Study Methodologies?
Recent advances in single-blind study methodologies focus on enhancing the rigor, transparency, and ethical conduct of research. These advances include improved randomization techniques, enhanced blinding methods, and the use of technology to minimize bias.
10.1. Improved Randomization Techniques
Randomization is a cornerstone of single-blind studies, and recent advances have focused on refining randomization techniques to minimize selection bias. These include the use of computer-generated random sequences, stratified randomization, and minimization techniques. Stratified randomization ensures that important characteristics are balanced across treatment groups, while minimization techniques dynamically adjust treatment assignments to maintain balance.
10.2. Enhanced Blinding Methods
While single-blind studies involve blinding participants, recent advances have focused on enhancing blinding methods to reduce the potential for unblinding. This includes the use of placebo treatments that closely resemble the active treatment in terms of appearance, taste, and smell. Researchers are also using techniques to assess the effectiveness of blinding, such as asking participants to guess which treatment they received.
10.3. Use Of Technology To Minimize Bias
Technology is playing an increasing role in minimizing bias in single-blind studies. This includes the use of electronic data capture systems to reduce data entry errors and standardize data collection. Researchers are also using automated outcome assessment tools to minimize assessment bias. For example, wearable sensors can be used to objectively measure physical activity levels, reducing the need for subjective self-reports.
10.4. Enhanced Transparency
Transparency is essential for maintaining trust and integrity in research. Recent advances have focused on enhancing transparency in single-blind studies. This includes registering clinical trials in public databases, such as ClinicalTrials.gov, and publishing detailed study protocols before the start of the study. Researchers are also encouraged to share their data and analysis code with other researchers to promote reproducibility and collaboration.
10.5. Ethical Considerations
Ethical considerations are paramount in single-blind studies, and recent advances have focused on enhancing the ethical conduct of research. This includes obtaining informed consent from participants, minimizing risks, and protecting confidentiality. Researchers are also encouraged to engage with patient advocacy groups and community stakeholders to ensure that their research is aligned with the needs and values of the communities they serve.
10.6. Application Of AI
Artificial intelligence (AI) is increasingly being used to analyze data in clinical trials. AI algorithms can help identify patterns and predict outcomes more accurately than traditional statistical methods.
11. What Are The Key Challenges In Conducting Single-Blind Studies?
Conducting single-blind studies presents several challenges that researchers must address to ensure the validity and reliability of their findings. These challenges range from maintaining effective blinding to managing ethical considerations and minimizing bias.
11.1. Maintaining Effective Blinding
One of the primary challenges in single-blind studies is maintaining effective blinding of participants. It is essential to ensure that participants remain unaware of whether they are receiving the active treatment or a placebo. This can be difficult if the treatment has noticeable side effects or if participants have prior knowledge about the treatments being compared.
11.2. Managing Ethical Considerations
Ethical considerations are paramount in single-blind studies, and researchers must navigate several ethical challenges. This includes obtaining informed consent from participants, minimizing risks, and protecting confidentiality. Researchers must also ensure that the study is conducted in accordance with ethical guidelines and regulations.
11.3. Minimizing Bias
Minimizing bias is a key challenge in single-blind studies, particularly observer bias. Since the researchers are aware of which treatment each participant is receiving, they may unintentionally influence the results. This can occur through subtle differences in how they interact with participants, how they collect data, or how they interpret the findings. Researchers should take steps to minimize the potential for bias, such as using standardized protocols, blinding outcome assessors, and conducting sensitivity analyses.
11.4. Resource Constraints
Conducting single-blind studies can be resource-intensive, particularly if the study involves a large number of participants or requires complex interventions. Researchers may face challenges related to funding, personnel, and equipment. It is essential to carefully plan the study and allocate resources efficiently to ensure that the study can be conducted successfully.
11.5. Patient Recruitment
Patient recruitment can be a significant challenge in single-blind studies. Researchers may struggle to recruit enough participants who meet the eligibility criteria and are willing to participate in the study. This can be particularly challenging if the study involves a rare condition or if the study requires participants to undergo invasive procedures. Researchers should develop a comprehensive recruitment plan and use a variety of strategies to reach potential participants.
12. What Is The Future Of Single-Blind Randomized Comparative Studies?
The future of single-blind randomized comparative studies involves several promising trends, including the use of personalized medicine, the integration of real-world data, and the development of adaptive trial designs.
12.1. Personalized Medicine
Personalized medicine, also known as precision medicine, involves tailoring medical treatments to the individual characteristics of each patient. In the future, single-blind studies are likely to play an increasing role in evaluating the effectiveness of personalized medicine interventions. This may involve using genetic information, biomarkers, and other individual characteristics to identify patients who are most likely to benefit from a particular treatment.
12.2. Integration Of Real-World Data
Real-world data (RWD) refers to data that are collected outside of traditional clinical trial settings. This includes data from electronic health records, insurance claims, and patient registries. In the future, single-blind studies are likely to be integrated with RWD to provide a more comprehensive picture of the treatment effects. RWD can be used to supplement the data collected in the trial and to assess the generalizability of the findings to real-world settings.
12.3. Adaptive Trial Designs
Adaptive trial designs allow researchers to modify the study protocol based on the data that are collected during the trial. This can include modifying the sample size, changing the treatment arms, or stopping the trial early if the treatment is found to be ineffective. Adaptive trial designs can make single-blind studies more efficient and flexible, allowing researchers to learn more from the data.
12.4. Enhanced Data Analysis Techniques
Advanced statistical methods, such as machine learning and artificial intelligence (AI), are increasingly being used to analyze data from single-blind studies. These methods can help identify patterns and predict outcomes more accurately than traditional statistical methods. AI can also be used to automate certain tasks, such as data entry and outcome assessment, reducing the potential for human error.
12.5. Patient-Centered Outcomes
There is a growing emphasis on measuring patient-centered outcomes in clinical trials. Patient-centered outcomes are those that are important to patients, such as quality of life, symptom relief, and functional status. In the future, single-blind studies are likely to focus more on measuring patient-centered outcomes to provide a more complete picture of the treatment effects.
Choosing the right study design is crucial for reliable research. At COMPARE.EDU.VN, we help you evaluate your options by providing comprehensive comparisons and insightful analyses.
Making sense of complex comparisons shouldn’t be a struggle. Visit COMPARE.EDU.VN for easy-to-understand analyses and detailed evaluations that empower you to make the right choices. For any questions, reach out to us at 333 Comparison Plaza, Choice City, CA 90210, United States, or contact us via Whatsapp at +1 (626) 555-9090. Visit our website at compare.edu.vn for more information.
FAQ: Single-Blind Randomized Comparative Studies
1. What is the primary purpose of a single-blind randomized comparative study?
The primary purpose is to evaluate the efficacy of a treatment while minimizing participant bias by keeping participants unaware of their treatment assignment.
2. How does randomization help in a single-blind study?
Randomization minimizes selection bias by ensuring each participant has an equal chance of being assigned to any treatment group, enhancing the validity of the study.
3. What are the main ethical considerations in conducting a single-blind study?
Key ethical considerations include obtaining informed consent, minimizing risks, maintaining confidentiality, and ensuring transparency throughout the research process.
4. What is the key difference between single-blind and double-blind studies?
The key difference is that in a single-blind study, only the participants are blinded, whereas in a double-blind study, both the participants and the researchers are blinded.
5. How can observer bias affect the results of a single-blind study?
Observer bias can occur when researchers unintentionally influence the results due to their awareness of treatment assignments, affecting data collection and interpretation.
6. What methods are used to analyze data in a single-blind randomized comparative study?
Data analysis typically includes descriptive statistics, inferential statistics, intention-to-treat analysis, per-protocol analysis, and subgroup analysis.
7. What are some examples of medical research that utilize single-blind studies?
Examples include studies in pain management, mental health interventions, rehabilitation techniques, surgical interventions, and vaccine trials.
8. What recent advances have been made in single-blind study methodologies?
Recent advances include improved randomization techniques, enhanced blinding methods, the use of technology to minimize bias, and enhanced transparency.
9. What are the key challenges in conducting single-blind studies?
Key challenges include maintaining effective blinding, managing ethical considerations, minimizing bias, addressing resource constraints, and ensuring successful patient recruitment.
10. How might single-blind studies evolve in the future?
Future trends include the use of personalized medicine approaches, integration of real-world data, development of adaptive trial designs, and enhanced data analysis techniques.