Do you need to compare two FTIR spectra accurately? Understanding How To Compare Two Ftir Spectra is crucial for product quality, safety, and research. At COMPARE.EDU.VN, we offer detailed guidance and tools to master spectral comparisons, ensuring you achieve accurate and reliable results. Dive into the essentials of spectral comparisons and discover the resources available at COMPARE.EDU.VN to enhance your analytical skills and decision-making. Explore advanced spectroscopic analysis and chemical compound identification today.
1. What is FTIR Spectroscopy and Why is Spectral Comparison Important?
FTIR spectroscopy, or Fourier Transform Infrared spectroscopy, is a technique used to obtain an infrared spectrum of absorption or emission of a solid, liquid, or gas. The FTIR spectrometer simultaneously collects high-spectral-resolution data over a wide spectral range. This confers a significant advantage over a dispersive spectrometer, which measures intensity over a narrow range of wavelengths at a time.
- Identifying Materials: FTIR is used to identify unknown materials.
- Quality Control: It ensures the consistency and quality of products.
- Research: It helps in analyzing chemical compositions and structures.
Spectral comparison is crucial because it allows you to determine if two samples are the same or different. By comparing an unknown sample’s spectrum to a known reference spectrum, you can identify the components of the unknown sample. According to research from the University of California, Los Angeles, accurate spectral comparison is essential in pharmaceutical quality control, ensuring that raw materials meet required standards.
2. What Are the Key Steps in Comparing Two FTIR Spectra?
Comparing two FTIR spectra involves several critical steps to ensure accuracy and reliability. These steps include data acquisition, baseline correction, normalization, peak identification, and qualitative and quantitative analysis.
- Data Acquisition: Use FTIR to obtain spectra of both the known reference and the unknown sample. Ensure consistent scanning parameters.
- Baseline Correction: Correct spectra to remove baseline drift, enhancing data accuracy.
- Normalization: Normalize spectra to minimize variations, improving comparison.
- Peak Identification: Identify key peaks for functional group analysis.
- Qualitative Analysis: Compare peak positions and intensities for compound identification.
- Quantitative Analysis: Measure peak areas or heights for concentration determination.
3. What Are the Important Parameters to Control When Comparing Spectra?
To draw chemically legitimate conclusions from an identity test, it’s essential to ensure that the sample and reference are run under identical experimental conditions. This control ensures that any observed differences can be attributed to the samples and not to variations in the experiment. According to a study by the University of Michigan, controlling scanning parameters significantly improves the accuracy of FTIR spectral analysis.
- Scanning Parameters: Reproduce the number of scans, instrumental resolution, and apodization function.
- Sample Preparation Technique: Ensure identical preparation methods to avoid variations in spectral appearance.
- Instrument Used: Measure spectra on the same instrument to minimize instrument-specific noise and artifacts.
- Operator: Ideally, the same person should prepare and measure the spectra to reduce variability.
4. How Do Scanning Parameters Affect Spectral Appearance in FTIR?
Scanning parameters, such as instrumental resolution, number of scans, and apodization function, significantly influence the appearance of FTIR spectra. Instrumental resolution, in particular, has a pronounced effect.
- Instrumental Resolution: High resolution provides well-resolved peaks, while low resolution can result in broad, unresolved features.
- Number of Scans: Increasing the number of scans improves the signal-to-noise ratio, enhancing spectral clarity.
- Apodization Function: Different apodization functions affect peak shape and resolution, impacting the accuracy of spectral comparisons.
Example: The IR spectrum of water vapor measured at 4 cm-1 instrumental resolution shows well-resolved peaks, while the same spectrum measured at 32 cm-1 instrumental resolution appears as a broad, unresolved blob.
5. Why is Sample Preparation Technique Crucial in Spectral Comparison?
The sample preparation technique is a critical parameter to control because different techniques can cause spectra to look different. This is particularly important in FTIR, where methods like ATR (Attenuated Total Reflection) and transmission techniques can produce varying results.
- ATR vs. Transmission: ATR spectra show depth of penetration variations with wavenumber, affecting peak intensities. Transmission spectra do not have this issue, but require different sample preparation.
- KBr Pellets: Variability in KBr pellet preparation can lead to spectral differences.
- Liquid Films: Film thickness variations can affect peak intensities in liquid samples.
Example: A sucrose spectrum measured using ATR shows different peak sizes around 1000 and 3200 cm-1 due to the wavenumber-dependent depth of penetration.
6. How Does the Instrument Used Affect FTIR Spectral Comparisons?
Even when measuring the same sample, different FTIR instruments can produce slightly different spectra due to unique noise and artifacts. While modern instruments are designed to minimize these differences, they can still be noticeable.
- Instrument-Specific Noise: Each instrument adds unique noise patterns to spectra.
- Calibration Differences: Variations in instrument calibration can affect peak positions and intensities.
- Component Quality: Differences in the quality of instrument components, such as detectors and light sources, can influence spectral resolution and sensitivity.
Best Practice: To mitigate these issues, it is best to measure sample and reference spectra on the same instrument.
7. Why is the FTIR Operator a Factor in Spectral Comparison?
The operator is a factor because no two people do things exactly the same way, especially with manual sample preparation techniques. This variability can lead to differences in the measured spectra.
- KBr Pellet Preparation: Manual preparation of KBr pellets can vary significantly among operators.
- Liquid Film Thickness: Applying consistent liquid film thickness is challenging and can introduce variability.
- Instrument Settings: Differences in how operators set up and run the instrument can affect results.
Recommendation: Ensure that all operators are properly trained and follow a clearly written standard operating procedure to minimize variability.
8. How Can Spectral Comparisons Be Used for Identity Testing?
Spectral comparisons are extensively used in identity testing, particularly in industries such as pharmaceuticals, food, cosmetics, and nutraceuticals. Identity testing involves comparing the spectrum of an unknown material to a reference spectrum to confirm its identity.
- Raw Material Verification: Ensuring the correct identity of incoming raw materials.
- Product Consistency: Verifying that each batch of a product matches the reference standard.
- Counterfeit Detection: Identifying counterfeit or substandard products.
Example: In the pharmaceutical industry, spectral comparison is used to confirm that the active pharmaceutical ingredient (API) in a drug product is indeed the correct compound. According to a study by Harvard University, this type of testing is critical for patient safety and regulatory compliance.
9. What is the Role of Spectral Libraries in FTIR Analysis?
Spectral libraries are databases containing reference spectra of known compounds. These libraries are essential tools for identifying unknown substances by comparing their spectra to the library entries.
- Compound Identification: Matching unknown spectra to known compounds in the library.
- Mixture Analysis: Identifying components in a mixture by comparing the mixture spectrum to individual compound spectra.
- Quality Control: Confirming the identity of materials against library standards.
Common Libraries: Examples include the NIST (National Institute of Standards and Technology) library, the Aldrich library, and various commercial libraries.
10. What Are the Limitations of FTIR Spectral Comparison?
While FTIR spectral comparison is a powerful technique, it has certain limitations. These limitations include issues with complex mixtures, spectral resolution, and sample preparation.
- Complex Mixtures: Analyzing complex mixtures can be challenging due to overlapping peaks.
- Spectral Resolution: Limited spectral resolution can hinder the differentiation of closely related compounds.
- Sample Preparation: Inconsistent sample preparation can introduce errors and variability.
- Database Limitations: The accuracy of spectral matching depends on the comprehensiveness and accuracy of the reference spectral library.
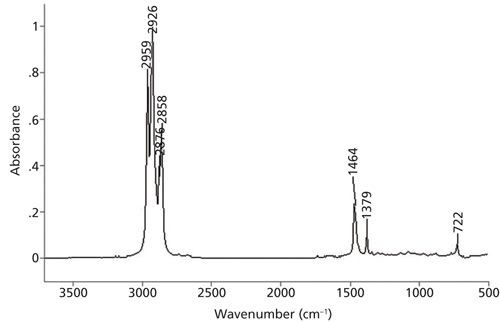
11. How Can FTIR Be Used to Determine Alkane Chain Length?
FTIR spectroscopy can be used to estimate the alkyl chain length in straight-chain alkanes by analyzing specific peaks and their intensities. This method leverages the unique vibrational modes of methyl (CH3) and methylene (CH2) groups.
- Methylene Rocking Peak: The presence of a methylene CH2 rocking peak at 720 ±10 cm-1 indicates alkyl chains with four or more methylenes in a row.
- CH2/CH3 Ratio: The ratio of CH2/CH3 peak intensities correlates to the methylene-to-methyl ratio, providing insight into chain length.
- C-H Stretching Peaks: The number of C-H stretching peaks between 3000 and 2850 cm-1 gives a general idea of alkyl chain length.
12. How Does the CH2/CH3 Ratio Correlate with Alkane Chain Length?
The ratio of methylene (CH2) to methyl (CH3) peak intensities in FTIR spectra correlates with the alkane chain length. This correlation is due to the fixed number of methyl groups (two) in straight-chain alkanes, allowing their peak intensities to serve as an internal standard.
- Low CH2/CH3 Ratio: Indicates shorter chain lengths, with methyl peaks being larger than methylene peaks.
- High CH2/CH3 Ratio: Indicates longer chain lengths, where methylene peaks are much larger and may mask the methyl peaks.
- Calibration Curves: Measuring known alkanes and plotting peak intensity ratios versus CH2/CH3 ratios can create calibration curves for quantitative analysis.
13. What Are Some Common Applications of FTIR Spectral Comparison?
FTIR spectral comparison is used in a wide range of applications across various industries. These applications leverage the technique’s ability to identify materials and ensure quality control.
- Pharmaceuticals: Identity testing of raw materials, verification of drug products, and detection of counterfeit drugs.
- Food and Beverage: Quality control of food ingredients, identification of contaminants, and verification of product authenticity.
- Cosmetics: Ensuring the quality and consistency of cosmetic ingredients and products.
- Environmental Monitoring: Identifying pollutants in air, water, and soil samples.
- Materials Science: Analyzing the composition and structure of polymers, composites, and other materials.
- Forensic Science: Identifying unknown substances in criminal investigations.
14. How Can You Normalize FTIR Spectra for Accurate Comparison?
Normalization is a critical step in preparing FTIR spectra for comparison. It involves adjusting the spectra to a common scale, minimizing variations due to differences in sample concentration, path length, or instrument response.
- Baseline Correction: Removing baseline drift caused by scattering or instrumental effects.
- Scaling to a Common Peak: Scaling the spectra based on the intensity of a reference peak.
- Vector Normalization: Dividing each data point by the norm of the entire spectrum.
- Min-Max Normalization: Scaling the data to a range between 0 and 1.
15. What is Baseline Correction and Why is it Important?
Baseline correction is the process of removing baseline drift or offset from FTIR spectra. Baseline drift can be caused by factors such as scattering, detector response, or sample thickness variations.
- Improving Accuracy: Correcting baseline drift improves the accuracy of peak intensity measurements.
- Enhancing Visual Comparison: A flat baseline makes it easier to visually compare spectra.
- Automated Analysis: Proper baseline correction is essential for automated spectral analysis and library searching.
16. How Do You Identify Key Peaks in FTIR Spectra for Comparison?
Identifying key peaks in FTIR spectra is essential for determining the functional groups present in a sample. Key peaks are typically those with high intensity and distinctive positions.
- Functional Group Regions: Familiarize yourself with the characteristic absorption regions for different functional groups (e.g., O-H, C=O, N-H).
- Peak Tables: Create a table of peak positions and intensities for easy reference.
- Reference Spectra: Compare the unknown spectrum to reference spectra of known compounds to identify key peaks.
- Software Tools: Use software tools that automatically identify and label peaks in the spectrum.
17. What Software Tools Are Available for FTIR Spectral Comparison?
Several software tools are available for FTIR spectral comparison, offering features such as automated peak identification, library searching, and spectral manipulation.
- Thermo Scientific OMNIC: Comprehensive software with advanced spectral analysis and library searching capabilities.
- Bruker OPUS: Powerful software for data acquisition, processing, and analysis.
- PerkinElmer Spectrum: User-friendly software for spectral analysis and comparison.
- NIST Chemistry WebBook: Online database with reference spectra and chemical information.
- Bio-Rad KnowItAll: Extensive spectral library with advanced search capabilities.
18. What is the Best Way to Visually Compare Two FTIR Spectra?
Visual comparison of FTIR spectra involves overlaying the spectra and examining differences in peak positions, intensities, and shapes.
- Overlaying Spectra: Plot the spectra on the same graph for direct comparison.
- Peak Positions: Compare the positions of key peaks to identify shifts or differences.
- Peak Intensities: Compare the relative intensities of peaks to assess differences in concentration or composition.
- Peak Shapes: Examine peak shapes for broadening or asymmetry, which may indicate differences in sample properties.
- Zooming In: Zoom in on specific regions of the spectrum to examine fine details.
19. How Do You Interpret Differences in Peak Intensities When Comparing FTIR Spectra?
Differences in peak intensities between two FTIR spectra can indicate variations in concentration, sample thickness, or the presence of different components.
- Concentration Differences: Higher peak intensities generally indicate higher concentrations of the corresponding component.
- Sample Thickness: Variations in sample thickness can affect peak intensities.
- Component Variations: Differences in peak intensities can indicate the presence of different components or impurities.
- Normalization: Always normalize the spectra before comparing peak intensities to account for variations in sample preparation or instrument response.
20. What is the Significance of Peak Splitting in FTIR Spectra?
Peak splitting in FTIR spectra can indicate the presence of multiple phases or crystalline forms in a sample. It can also result from Fermi resonance, where two vibrational modes interact and split into two peaks.
- Crystalline Forms: Different crystalline forms of a compound can exhibit different peak splitting patterns.
- Fermi Resonance: Interaction between vibrational modes can cause peaks to split.
- Solid State Effects: Peak splitting is more common in solid samples due to intermolecular interactions.
21. How Can I Ensure the Accuracy of My FTIR Spectral Comparisons?
Ensuring the accuracy of FTIR spectral comparisons involves careful attention to experimental details, proper data processing, and thorough analysis.
- Calibration: Regularly calibrate the FTIR instrument using a known standard.
- Quality Control: Run quality control samples to verify instrument performance.
- Standard Operating Procedures: Follow clearly written standard operating procedures for sample preparation and data acquisition.
- Data Processing: Use appropriate data processing techniques, such as baseline correction and normalization.
- Expert Review: Have an experienced spectroscopist review your data and analysis.
22. What Are the Ethical Considerations in Spectral Comparison?
When performing spectral comparisons, particularly in regulated industries, ethical considerations are paramount to ensure data integrity and prevent fraudulent activities.
- Data Manipulation: Avoid manipulating data to achieve desired results.
- Transparency: Clearly document all data processing steps and any deviations from standard operating procedures.
- Reproducibility: Ensure that your results are reproducible by other researchers.
- Conflict of Interest: Disclose any potential conflicts of interest that may bias your analysis.
23. What are Advanced Techniques for Analyzing FTIR Spectra?
Beyond basic spectral comparison, advanced techniques can provide deeper insights into material composition and structure.
- Deconvolution: Resolving overlapping peaks to improve spectral resolution.
- Curve Fitting: Modeling peak shapes to quantify individual components in a spectrum.
- Principal Component Analysis (PCA): Reducing the dimensionality of spectral data for pattern recognition and classification.
- Two-Dimensional Correlation Spectroscopy (2D-COS): Enhancing spectral resolution and identifying interactions between vibrational modes.
24. How to Interpret the Results of a Spectral Comparison?
Interpreting the results of a spectral comparison requires careful consideration of peak positions, intensities, and shapes. A close match between the sample spectrum and the reference spectrum indicates a high degree of similarity between the two materials.
- Peak Matching: Compare the positions and intensities of key peaks in the sample spectrum to those in the reference spectrum.
- Quantitative Analysis: Use peak areas or heights to quantify the concentration of components in the sample.
- Statistical Analysis: Use statistical methods, such as correlation analysis or principal component analysis, to compare spectra and identify differences.
25. What are the Common Mistakes to Avoid When Comparing Spectra?
Avoiding common mistakes in FTIR spectral comparison is crucial for obtaining accurate and reliable results.
- Neglecting Baseline Correction: Failing to correct for baseline drift can lead to inaccurate peak intensity measurements.
- Ignoring Resolution Effects: Ignoring the effects of instrumental resolution on peak shapes can result in misidentification of compounds.
- Poor Sample Preparation: Inconsistent sample preparation can introduce variability and errors in spectral comparisons.
- Overlooking Water Vapor: Water vapor in the instrument or sample can produce strong absorption bands that interfere with spectral analysis.
26. What are the Safety Precautions to Take When Working with FTIR Spectrometers?
When working with FTIR spectrometers, it is essential to follow safety precautions to protect yourself and prevent accidents.
- Laser Safety: Be aware of the potential hazards associated with the laser source in the spectrometer.
- Chemical Safety: Follow proper handling procedures for any chemicals used in sample preparation.
- Electrical Safety: Ensure that the instrument is properly grounded and that all electrical connections are secure.
- Ventilation: Work in a well-ventilated area to avoid exposure to harmful vapors.
- Personal Protective Equipment (PPE): Wear appropriate PPE, such as gloves and eye protection, when handling samples and chemicals.
27. How Can Spectroscopy Contribute to Environmental Monitoring?
Spectroscopy is a powerful tool for environmental monitoring, providing rapid and sensitive detection of pollutants in air, water, and soil.
- Air Quality Monitoring: Measuring the concentration of pollutants such as ozone, nitrogen dioxide, and particulate matter.
- Water Quality Monitoring: Detecting contaminants such as heavy metals, pesticides, and organic compounds.
- Soil Analysis: Determining the composition and nutrient content of soil samples.
- Remote Sensing: Monitoring environmental conditions from satellites or aircraft.
28. What Future Trends Can We Expect in FTIR Spectroscopic Analysis?
FTIR spectroscopy is a constantly evolving field, with new techniques and applications emerging all the time.
- Miniaturization: Development of smaller, more portable FTIR instruments.
- Hyperspectral Imaging: Combining FTIR spectroscopy with imaging techniques to provide spatial and spectral information.
- Artificial Intelligence: Using AI and machine learning to automate spectral analysis and improve accuracy.
- New Materials: Applying FTIR spectroscopy to the characterization of new materials such as nanomaterials and biomaterials.
29. How Can You Troubleshoot Common Issues in FTIR Spectroscopy?
Troubleshooting common issues in FTIR spectroscopy can help you obtain accurate and reliable results.
- Poor Signal-to-Noise Ratio: Increase the number of scans, optimize instrument settings, or clean the instrument components.
- Baseline Drift: Correct for baseline drift using appropriate data processing techniques.
- Water Vapor Interference: Purge the instrument with dry air or use a desiccant to remove water vapor.
- Sample Contamination: Use clean sample holders and follow proper sample preparation procedures.
30. What are the Different Sampling Techniques Used in FTIR Spectroscopy?
Different sampling techniques are used in FTIR spectroscopy to accommodate various types of samples.
- Transmission: Passing the IR beam through a sample. This technique is suitable for liquids, gases, and thin films.
- Attenuated Total Reflection (ATR): Measuring the changes that occur when an IR beam is reflected off the surface of a sample. This technique is suitable for solids, liquids, and pastes.
- Diffuse Reflectance: Measuring the IR radiation that is scattered from a rough surface. This technique is suitable for powders and heterogeneous materials.
- Specular Reflectance: Measuring the IR radiation that is reflected from a smooth surface. This technique is suitable for coatings and thin films.
Conclusion
Mastering how to compare two FTIR spectra is essential for ensuring product quality, conducting accurate research, and making informed decisions. By controlling experimental variables, utilizing spectral libraries, and employing advanced analysis techniques, you can achieve reliable results. For more comprehensive comparisons and resources, visit COMPARE.EDU.VN, where we provide the tools and information you need to excel in spectral analysis. Explore, compare, and decide with confidence at COMPARE.EDU.VN.
For more information, contact us at:
Address: 333 Comparison Plaza, Choice City, CA 90210, United States
Whatsapp: +1 (626) 555-9090
Website: compare.edu.vn
FAQ: Frequently Asked Questions About FTIR Spectral Comparison
Q1: What is the most important factor when comparing two FTIR spectra?
Answer: Controlling experimental conditions and scanning parameters to ensure consistency between measurements.
Q2: How can I normalize my FTIR spectra effectively?
Answer: Use baseline correction and scale the spectra based on a reference peak or vector normalization.
Q3: What should I do if I encounter baseline drift in my FTIR spectra?
Answer: Apply baseline correction algorithms in your data processing software.
Q4: How do I identify key peaks in FTIR spectra for comparison?
Answer: Familiarize yourself with characteristic absorption regions and compare your spectra to reference standards.
Q5: Which software tools are best for comparing FTIR spectra?
Answer: Thermo Scientific OMNIC, Bruker OPUS, and PerkinElmer Spectrum are excellent choices.
Q6: What safety precautions should I take when working with an FTIR spectrometer?
Answer: Be aware of laser safety, use proper chemical handling procedures, and ensure electrical safety.
Q7: How can FTIR spectroscopy be used in environmental monitoring?
Answer: It can detect pollutants in air, water, and soil samples, providing valuable environmental data.
Q8: How can I improve the signal-to-noise ratio in my FTIR spectra?
Answer: Increase the number of scans, optimize instrument settings, and clean the instrument components.
Q9: What are the limitations of using spectral libraries for comparison?
Answer: The accuracy depends on the comprehensiveness and accuracy of the library’s reference spectra.
Q10: How can FTIR be used to determine alkane chain length?
Answer: By analyzing the methylene rocking peak and the CH2/CH3 ratio in the spectra.
This image illustrates an FTIR spectrum, showcasing a visual representation of data used for spectral comparison, aiding in the identification and analysis of chemical compounds.
This diagram represents the chemical structure of octane, demonstrating the arrangement of carbon and hydrogen atoms, which is essential for understanding its spectral properties in FTIR analysis.
This figure highlights the peaks in an FTIR spectrum of octane, showing the specific absorption patterns used to identify the presence and characteristics of this alkane.
This illustrates the impact of instrumental resolution on spectral appearance, with high resolution showing well-resolved peaks and low resolution resulting in broad, unresolved features.
This image demonstrates the effect of different sample preparation techniques on FTIR spectra, highlighting variations in peak intensities and shapes due to ATR and non-ATR methods.
This shows the FTIR spectrum of petroleum jelly, illustrating the characteristic peaks of long-chain alkanes and the masking of methyl peaks due to intense methylene peaks.
This demonstrates the asymmetric methyl and methylene stretching peaks for ethyl groups and alkanes, showing how the CH2/CH3 ratio correlates with chain length.
This illustrates the spectral analysis of different chemical compounds, showcasing the unique absorption patterns that allow for compound identification and comparison.
This shows the peak assignments in a spectrum, used to determine the functional groups present and propose a chemical structure.
A professional portrait of Brian C. Smith, Senior Infrared Product Specialist for PerkinElmer, highlighting his expertise in FT-IR analyses and infrared spectral interpretation.