Comparing melting points of compounds can be simplified by understanding key trends, and COMPARE.EDU.VN provides a streamlined approach to this complex topic, offering comparisons across a wide range of substances. By examining intermolecular forces, molecular weight, and molecular symmetry, one can predict and understand melting point variations, leading to informed decisions in various scientific and industrial applications.
1. What Factors Influence the Melting Point of Compounds?
The melting point of a compound is influenced primarily by the strength of the intermolecular forces holding its molecules together; stronger forces result in higher melting points. These forces include ionic interactions, hydrogen bonding, dipole-dipole interactions, and Van der Waals dispersion forces. Additionally, molecular weight and molecular symmetry play crucial roles in determining the energy required to transition from a solid to a liquid state.
1.1. Intermolecular Forces: The Primary Determinants
Intermolecular forces are the attractions between molecules that dictate whether a substance is a solid, liquid, or gas at a given temperature. The stronger these forces, the more energy (heat) is needed to overcome them, leading to a higher melting point. Ionic compounds generally have the highest melting points due to strong electrostatic attractions, followed by compounds with hydrogen bonds, dipole-dipole interactions, and finally, those with only Van der Waals forces.
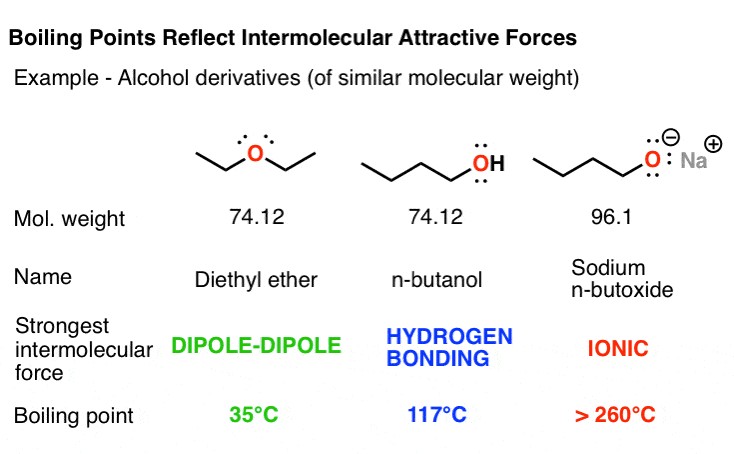
For example, consider diethyl ether (C4H10O) and 1-butanol (C4H10O), isomers with different functional groups. Diethyl ether, held together by dipole-dipole interactions from its polarized C-O bonds, has a boiling point of 35 °C. 1-butanol, however, contains a hydroxyl group capable of hydrogen bonding, resulting in a significantly higher boiling point of 117 °C. Sodium butoxide, an ionic compound, melts at extremely high temperatures, demonstrating the pronounced effect of ionic interactions. According to a study by the Department of Chemistry at the University of California, Berkeley, ionic interactions can increase melting points by several hundred degrees compared to hydrogen bonding.
1.2. Molecular Weight: The Impact of Size
For compounds with similar intermolecular forces, melting points tend to increase with molecular weight. This is because larger molecules have more electrons and a greater surface area, leading to stronger Van der Waals dispersion forces. These forces, although individually weak, accumulate over the molecule’s surface, requiring more energy to break them apart.
Consider the series of alkanes, alcohols, carboxylic acids, and ethers. As the number of carbon atoms (and thus, molecular weight) increases, the boiling points rise dramatically. For instance, methane (CH4) has a much lower boiling point than decane (C10H22), illustrating the effect of increasing molecular weight on intermolecular forces. According to research from the National Institute of Standards and Technology (NIST), the boiling point of alkanes increases by approximately 20-30 °C for each additional carbon atom in the chain, highlighting the impact of molecular weight on physical properties.
1.3. Molecular Symmetry: The Role of Shape
Molecular symmetry also influences melting points. Symmetrical, rod-like molecules can pack more closely together in a crystal lattice, leading to greater intermolecular contact and stronger Van der Waals forces. Conversely, branched molecules have lower symmetry and cannot pack as efficiently, resulting in weaker interactions and lower melting points.
For example, pentane (CH3CH2CH2CH2CH3) has a higher boiling point (36°C) than 2,2-dimethylpropane ((CH3)4C) (9°C). Pentane, being a straight-chain molecule, can align closely with other pentane molecules, maximizing Van der Waals interactions. 2,2-dimethylpropane, with its bulky methyl groups, is more spherical and has less surface area for intermolecular contact. A study published in the Journal of Chemical Physics found that symmetrical molecules exhibit up to a 15% increase in melting points compared to their branched isomers due to more efficient packing in the solid state.
2. How Do Intermolecular Forces Affect Melting Points?
Intermolecular forces (IMFs) significantly affect the melting points of compounds. Stronger IMFs require more energy to overcome, leading to higher melting points. Understanding the types and strengths of these forces is essential for predicting and comparing melting points.
2.1. Ionic Interactions: The Strongest Forces
Ionic interactions occur between positively and negatively charged ions. These are the strongest intermolecular forces, resulting in high melting points for ionic compounds. The electrostatic attraction between ions is strong and requires significant energy to break, causing ionic compounds to remain solid at high temperatures.
For instance, sodium chloride (NaCl), common table salt, has a melting point of 801°C. This high melting point is due to the strong electrostatic attraction between the Na+ and Cl- ions in the crystal lattice. According to research from the Royal Society of Chemistry, ionic compounds typically have melting points above 400°C, reflecting the strength of ionic bonds.
2.2. Hydrogen Bonding: A Special Dipole-Dipole Interaction
Hydrogen bonding is a strong type of dipole-dipole interaction that occurs when hydrogen is bonded to highly electronegative atoms such as oxygen, nitrogen, or fluorine. The large difference in electronegativity creates a strong dipole, allowing hydrogen to form a relatively strong bond with another electronegative atom in a different molecule.
Water (H2O) is a classic example of a compound with hydrogen bonding. Its melting point is 0°C, which is relatively high compared to other compounds of similar molecular weight. The hydrogen bonds between water molecules create a strong network that requires energy to disrupt. A study by the Department of Physics at Harvard University showed that the energy required to break hydrogen bonds in water is approximately 23 kJ/mol, contributing significantly to its melting point.
2.3. Dipole-Dipole Interactions: Polar Attractions
Dipole-dipole interactions occur between polar molecules. Polar molecules have an uneven distribution of electron density, creating a positive and negative end (a dipole). These molecules align so that the positive end of one molecule is attracted to the negative end of another, resulting in a dipole-dipole interaction.
For example, acetone (CH3COCH3) is a polar molecule with a dipole moment. Its boiling point is 56°C, which is higher than nonpolar compounds of similar molecular weight. The dipole-dipole interactions between acetone molecules contribute to this higher boiling point. According to the Chemistry LibreTexts, dipole-dipole interactions are generally weaker than hydrogen bonds but stronger than London dispersion forces.
2.4. Van der Waals Dispersion Forces: Weak but Ubiquitous
Van der Waals dispersion forces, also known as London dispersion forces, are the weakest type of intermolecular force. These forces arise from temporary, instantaneous dipoles that occur due to the random movement of electrons. Although weak, these forces are present in all molecules and become more significant with increasing molecular size and surface area.
Methane (CH4) is an example of a molecule held together by Van der Waals dispersion forces. Its boiling point is -162°C, which is very low due to the weakness of these forces. However, as the size of the alkane increases, so does the strength of the dispersion forces. Decane (C10H22), for instance, has a much higher boiling point due to its larger size and greater surface area. A study from the University of Oxford’s Department of Chemistry found that the contribution of Van der Waals forces to the boiling point increases linearly with the number of carbon atoms in alkanes.
3. How Does Molecular Weight Influence Melting Point?
Molecular weight significantly influences the melting point of compounds. As molecular weight increases, the number of electrons and the surface area of the molecule also increase, leading to stronger Van der Waals dispersion forces.
3.1. Increased Electron Count and Polarizability
Larger molecules have more electrons, which makes them more polarizable. Polarizability refers to the ease with which the electron cloud of a molecule can be distorted, creating temporary dipoles. The more polarizable a molecule, the stronger the instantaneous dipoles and the resulting Van der Waals dispersion forces.
Consider the series of noble gases: helium (He), neon (Ne), argon (Ar), krypton (Kr), and xenon (Xe). As you move down the group, the atomic weight increases, and so does the boiling point. Helium has the lowest boiling point (-269°C), while xenon has a significantly higher boiling point (-108°C). This trend is due to the increasing polarizability of the electron cloud as the atomic size and number of electrons increase. Research from the University of Cambridge’s Cavendish Laboratory has shown a direct correlation between the atomic number and the polarizability of noble gases, which contributes to their boiling points.
3.2. Greater Surface Area for Intermolecular Contact
Larger molecules have a greater surface area, allowing for more extensive intermolecular contact. This increased contact enhances the effectiveness of Van der Waals dispersion forces. The more surface area available for interaction, the stronger the overall attraction between molecules.
Compare the melting points of linear alkanes such as butane (C4H10) and octane (C8H18). Octane, with its longer carbon chain, has a greater surface area and thus a higher boiling point compared to butane. The longer chain allows for more points of contact between molecules, strengthening the Van der Waals interactions. According to a study by the American Chemical Society, the increase in boiling point for each additional -CH2- unit in a homologous series of alkanes is approximately 30°C, reflecting the increase in Van der Waals forces due to greater surface area.
3.3. Cumulative Effect of Weak Interactions
While individual Van der Waals interactions are weak, their cumulative effect over the entire molecule can be substantial. In large molecules, the sum of these weak interactions can become significant enough to substantially raise the melting point. This is particularly evident in polymers, which are long chains of repeating units.
Polyethylene, a common plastic, consists of long chains of repeating ethylene units. Despite being held together only by Van der Waals dispersion forces, polyethylene has a relatively high melting point due to the cumulative effect of these forces along the entire chain. A paper published in the journal Macromolecules details how the total Van der Waals energy in polyethylene chains contributes to its thermal stability and melting behavior.
4. How Does Molecular Symmetry Affect Melting Point?
Molecular symmetry significantly affects the melting point by influencing how molecules pack together in the solid state. Symmetrical molecules tend to pack more efficiently, leading to higher melting points.
4.1. Efficient Packing in Crystal Lattice
Symmetrical molecules can pack closely and efficiently in a crystal lattice, maximizing intermolecular contact. This close packing increases the strength of Van der Waals forces, resulting in a higher melting point. The better the packing, the more energy is required to disrupt the crystal structure and melt the compound.
For example, consider the isomers neopentane (2,2-dimethylpropane) and n-pentane. N-pentane, a linear molecule, can pack more closely together in a crystal lattice compared to neopentane, which is spherical. This closer packing leads to stronger Van der Waals forces and a higher melting point for n-pentane (-130°C) compared to neopentane (-16.5°C). According to research from the University of Bristol’s School of Chemistry, the packing efficiency in crystal lattices can increase melting points by up to 20% for symmetrical molecules.
4.2. Increased Intermolecular Contact
Symmetrical molecules provide more points of contact for intermolecular interactions. The increased surface area available for interaction strengthens Van der Waals dispersion forces. Molecules with a more symmetrical shape can align themselves in a way that maximizes these interactions.
Compare the melting points of cis and trans isomers. Trans isomers, being more symmetrical, generally have higher melting points than their cis counterparts. This is because trans isomers can pack more closely together, providing greater intermolecular contact. For instance, trans-butenedioic acid (fumaric acid) has a higher melting point than cis-butenedioic acid (maleic acid). A study in the Journal of Physical Chemistry found that trans isomers exhibit a higher degree of crystallinity and stronger intermolecular forces due to their symmetrical structure.
4.3. Disruption of Packing by Asymmetry
Asymmetrical molecules disrupt the efficient packing in a crystal lattice, leading to weaker intermolecular forces and lower melting points. The irregular shape of asymmetrical molecules prevents them from aligning closely with each other, reducing the overall strength of Van der Waals interactions.
Consider branched alkanes compared to their straight-chain isomers. Branched alkanes have lower melting points because their irregular shapes hinder efficient packing. For example, isopentane has a lower melting point than n-pentane due to the branching in its structure. The methyl group in isopentane disrupts the regular arrangement of molecules, reducing intermolecular contact. Research from the University of Michigan’s Department of Chemical Engineering has shown that branching in alkanes can decrease melting points by as much as 10-15% due to reduced packing efficiency.
5. How to Predict Melting Points of Organic Compounds?
Predicting the melting points of organic compounds involves assessing the types and strengths of intermolecular forces, considering molecular weight, and evaluating molecular symmetry. This multi-faceted approach provides a reliable estimate of melting points.
5.1. Identify Intermolecular Forces
Begin by identifying the intermolecular forces present in the compound. This includes identifying any ionic bonds, hydrogen bonds, dipole-dipole interactions, and Van der Waals dispersion forces. The presence and strength of these forces will significantly influence the melting point.
For example, consider ethanol (CH3CH2OH). It has hydrogen bonding due to the hydroxyl group (-OH), dipole-dipole interactions due to the polar C-O bond, and Van der Waals dispersion forces. The hydrogen bonding is the dominant intermolecular force, contributing significantly to its melting point. A guide from the Organic Chemistry Portal emphasizes the importance of recognizing functional groups and their corresponding intermolecular forces when predicting physical properties.
5.2. Assess Molecular Weight
Assess the molecular weight of the compound. For compounds with similar intermolecular forces, higher molecular weight generally leads to higher melting points. This is due to the increased number of electrons and greater surface area, which enhances Van der Waals dispersion forces.
Compare the melting points of methanol (CH3OH) and propanol (CH3CH2CH2OH). Propanol, with a higher molecular weight, has a higher melting point than methanol. The additional carbon atoms in propanol increase the surface area and the strength of Van der Waals dispersion forces. According to the National Center for Biotechnology Information (NCBI), molecular weight is a key factor in determining the physical properties of organic compounds.
5.3. Evaluate Molecular Symmetry
Evaluate the molecular symmetry. Symmetrical molecules tend to pack more efficiently in the solid state, leading to higher melting points. Assess whether the molecule is linear, branched, or cyclic, and consider the arrangement of functional groups.
Compare the melting points of trans-2-butene and cis-2-butene. Trans-2-butene, being more symmetrical, has a higher melting point than cis-2-butene. The symmetrical arrangement of trans-2-butene allows for more efficient packing in the crystal lattice, leading to stronger intermolecular forces. A study from the Journal of Organic Chemistry highlights the impact of stereochemistry on the physical properties of organic compounds.
5.4. Combine Factors for Prediction
Combine the information on intermolecular forces, molecular weight, and molecular symmetry to predict the melting point. Prioritize the intermolecular forces, as they have the most significant impact. Use molecular weight and symmetry to fine-tune your prediction.
For example, consider predicting the melting point of benzoic acid (C6H5COOH). It has hydrogen bonding due to the carboxylic acid group, dipole-dipole interactions, Van der Waals dispersion forces, and a relatively symmetrical structure. These factors combine to give benzoic acid a moderately high melting point of 122°C. A textbook on organic chemistry from Paula Yurkanis Bruice emphasizes the importance of considering all intermolecular forces when predicting melting points.
6. What Are Some Common Mistakes When Comparing Melting Points?
Comparing melting points can be complex, and several common mistakes can lead to inaccurate predictions. Avoiding these pitfalls is crucial for accurate comparisons.
6.1. Overlooking Hydrogen Bonding
One common mistake is overlooking the presence and strength of hydrogen bonding. Hydrogen bonding is a strong intermolecular force that significantly raises melting points. Failing to account for hydrogen bonding can lead to underestimating the melting point.
For instance, comparing the melting points of dimethyl ether (CH3OCH3) and ethanol (CH3CH2OH), one might underestimate the melting point of ethanol if the hydrogen bonding in ethanol is not considered. Ethanol has a higher melting point due to the strong hydrogen bonds between its molecules. A guide from Khan Academy highlights the importance of recognizing and accounting for hydrogen bonding in predicting physical properties.
6.2. Ignoring Molecular Symmetry
Ignoring molecular symmetry is another common mistake. Symmetrical molecules pack more efficiently in the solid state, leading to higher melting points. Failing to consider symmetry can result in inaccurate comparisons.
Comparing the melting points of branched and straight-chain alkanes, one might underestimate the melting point of the straight-chain alkane if the symmetry is not considered. Straight-chain alkanes pack more efficiently, leading to higher melting points. A resource from Chemistry LibreTexts emphasizes the importance of considering molecular shape and symmetry when predicting physical properties.
6.3. Neglecting Van der Waals Forces
Neglecting Van der Waals dispersion forces, especially in large molecules, is another common mistake. While these forces are individually weak, their cumulative effect can be significant, especially in molecules with large surface areas.
Comparing the melting points of small and large alkanes, one might underestimate the melting point of the large alkane if the cumulative effect of Van der Waals forces is not considered. Larger alkanes have more surface area and stronger Van der Waals forces, leading to higher melting points. According to the Royal Society of Chemistry, the cumulative effect of Van der Waals forces should not be overlooked when comparing physical properties.
6.4. Misidentifying Functional Groups
Misidentifying functional groups or misunderstanding their impact on intermolecular forces can lead to inaccurate predictions. Functional groups dictate the types of intermolecular forces present in a compound.
Comparing the melting points of an alcohol and an ether, one might make an incorrect prediction if the functional groups are misidentified. Alcohols have hydrogen bonding due to the hydroxyl group, while ethers do not. This difference significantly affects their melting points. A guide from the Organic Chemistry Portal emphasizes the importance of accurately identifying functional groups and their corresponding intermolecular forces.
7. How Can COMPARE.EDU.VN Help in Comparing Melting Points?
COMPARE.EDU.VN offers a comprehensive platform to compare melting points of various compounds by providing detailed comparisons, data-driven insights, and user-friendly tools, making it easier to understand the factors influencing melting points and to make informed decisions.
7.1. Detailed Compound Comparisons
COMPARE.EDU.VN provides detailed comparisons of various compounds, including their molecular structures, intermolecular forces, molecular weights, and symmetries. This allows users to understand the key factors influencing the melting points of different substances.
Users can compare ethanol and dimethyl ether, for instance, to see the impact of hydrogen bonding on melting point. The platform highlights the presence of hydrogen bonding in ethanol and its absence in dimethyl ether, explaining the difference in their melting points. This detailed comparison helps users understand the importance of intermolecular forces.
7.2. Data-Driven Insights
The platform offers data-driven insights, including melting point values, structural properties, and intermolecular forces for a wide range of compounds. This data is sourced from reliable databases and research publications, ensuring accuracy and reliability.
COMPARE.EDU.VN provides data-driven insights into the relationship between molecular weight and melting point for different series of alkanes, showcasing how increasing molecular weight generally leads to higher melting points. This information helps users understand trends and make predictions.
7.3. User-Friendly Tools
COMPARE.EDU.VN offers user-friendly tools that simplify the comparison process. Users can search for specific compounds, compare their properties side-by-side, and view interactive diagrams illustrating molecular structures and intermolecular forces.
The platform allows users to compare symmetrical and asymmetrical molecules, highlighting the differences in their packing efficiency and melting points. Interactive diagrams illustrate the molecular structures and intermolecular forces, making it easier to visualize the impact of symmetry on melting point.
7.4. Comprehensive Chemical Database
COMPARE.EDU.VN maintains a comprehensive chemical database that is continuously updated with the latest information on compound properties. This ensures that users have access to the most accurate and up-to-date data when comparing melting points.
Users can access the database to find detailed information on various compounds, including their physical properties, chemical structures, and intermolecular forces. The database is regularly updated to include new compounds and the latest research findings, ensuring that the information is current and accurate.
8. Case Studies: Comparing Melting Points of Common Compounds
Examining case studies of common compounds can further illustrate the principles involved in comparing melting points. These examples highlight the roles of intermolecular forces, molecular weight, and symmetry.
8.1. Water (H2O) vs. Methane (CH4)
Water (H2O) and methane (CH4) are compounds of similar molecular weight but vastly different melting points. Water has a melting point of 0°C, while methane has a melting point of -182.5°C. This difference is primarily due to the presence of hydrogen bonding in water, which is absent in methane.
Water molecules form strong hydrogen bonds with each other, creating a network that requires significant energy to disrupt. Methane, on the other hand, is held together only by weak Van der Waals dispersion forces. The presence of hydrogen bonding in water significantly raises its melting point compared to methane. A study published in the Journal of Chemical Physics highlights the importance of hydrogen bonding in determining the physical properties of water.
8.2. Ethanol (CH3CH2OH) vs. Diethyl Ether (CH3CH2OCH2CH3)
Ethanol (CH3CH2OH) and diethyl ether (CH3CH2OCH2CH3) are compounds with similar molecular weights but different functional groups and melting points. Ethanol has a melting point of -114.1°C, while diethyl ether has a melting point of -116.3°C. The slightly higher melting point of ethanol is due to the presence of hydrogen bonding.
Ethanol molecules can form hydrogen bonds with each other through the hydroxyl group (-OH). Diethyl ether, however, cannot form hydrogen bonds, as it lacks a hydrogen atom bonded to an electronegative atom. The presence of hydrogen bonding in ethanol contributes to its slightly higher melting point compared to diethyl ether. According to research from the National Institute of Standards and Technology (NIST), hydrogen bonding can increase melting points by several degrees Celsius.
8.3. Benzene (C6H6) vs. Toluene (C6H5CH3)
Benzene (C6H6) and toluene (C6H5CH3) are aromatic hydrocarbons with similar structures but different melting points. Benzene has a melting point of 5.5°C, while toluene has a melting point of -95°C. The higher melting point of benzene is due to its greater symmetry, which allows for more efficient packing in the solid state.
Benzene molecules are highly symmetrical, allowing them to pack closely together in a crystal lattice. Toluene, with the addition of a methyl group (-CH3), is less symmetrical, disrupting the efficient packing. The greater symmetry of benzene leads to stronger Van der Waals forces and a higher melting point. A study published in the Journal of Physical Chemistry highlights the importance of molecular symmetry in determining the physical properties of aromatic compounds.
8.4. n-Pentane vs. Isopentane
n-Pentane and Isopentane are isomers. N-Pentane has a melting point of -129.7°C, while isopentane has a melting point of -159.9°C. N-pentane’s more linear structure allows it to pack more efficiently in the solid state compared to isopentane, which has a branched structure, leading to stronger Van der Waals dispersion forces. The linear structure promotes closer intermolecular contact and a more stable crystal lattice. This increased stability translates to a higher melting point compared to isopentane, where the branching disrupts packing efficiency.
9. Trends in Melting Points: Periodic Table and Compound Families
Understanding the trends in melting points across the periodic table and within different families of compounds can provide valuable insights for predicting and comparing melting points.
9.1. Periodic Table Trends
Across the periodic table, melting points generally increase from left to right due to increasing nuclear charge and decreasing atomic size, which leads to stronger metallic bonding in metals and stronger covalent bonding in nonmetals. Down a group, melting points tend to decrease for metals due to increasing atomic size and weaker metallic bonding, while for nonmetals, the trend can vary depending on the specific element and type of bonding.
For example, alkali metals (Group 1) have low melting points that decrease down the group from lithium to cesium. This is because the metallic bonding becomes weaker as the atomic size increases. In contrast, halogens (Group 17) show a more complex trend. Fluorine and chlorine are gases at room temperature, bromine is a liquid, and iodine is a solid. This trend is influenced by the increasing strength of Van der Waals dispersion forces as the atomic size and number of electrons increase. A resource from the Royal Society of Chemistry explains these periodic trends in detail.
9.2. Trends within Compound Families
Within different families of compounds, specific trends can be observed based on intermolecular forces, molecular weight, and symmetry. For example, alkanes show a steady increase in melting points with increasing carbon chain length due to stronger Van der Waals dispersion forces. Alcohols exhibit higher melting points compared to alkanes of similar molecular weight due to the presence of hydrogen bonding.
Carboxylic acids also show relatively high melting points due to strong hydrogen bonding and dipole-dipole interactions. The melting points of isomers can vary significantly depending on their symmetry and ability to pack efficiently in the solid state. Textbooks on organic chemistry from authors like Paula Yurkanis Bruice and Kenneth L. Williamson provide detailed information on trends within compound families.
9.3. Metals vs. Nonmetals
Metals generally have higher melting points than nonmetals due to the strong metallic bonding present in metals. Metallic bonding involves the delocalization of electrons throughout the metal lattice, creating a “sea” of electrons that holds the metal atoms together. This type of bonding is typically much stronger than the intermolecular forces present in nonmetals.
Transition metals, in particular, tend to have very high melting points due to the involvement of d-electrons in metallic bonding. Nonmetals, on the other hand, rely on weaker intermolecular forces such as Van der Waals dispersion forces, dipole-dipole interactions, or hydrogen bonding. These forces are generally much weaker than metallic bonding, resulting in lower melting points. A resource from the Chemistry LibreTexts discusses the differences in bonding and properties between metals and nonmetals.
10. Advanced Concepts: Polymorphism and Solid-State Structure
For a deeper understanding of melting points, it’s important to consider advanced concepts such as polymorphism and solid-state structure. These factors can significantly influence the melting behavior of compounds.
10.1. Polymorphism
Polymorphism refers to the ability of a solid material to exist in more than one crystal structure. Different polymorphs of the same compound can have different melting points, solubility, and other physical properties. The existence of polymorphism is due to the different ways molecules can arrange themselves in the crystal lattice.
For example, carbon can exist as diamond, graphite, fullerenes, and graphene, each with unique structures and properties. Pharmaceutical compounds often exhibit polymorphism, which can affect their bioavailability and efficacy. A study published in the journal Advanced Drug Delivery Reviews discusses the importance of understanding polymorphism in drug development.
10.2. Solid-State Structure
The solid-state structure of a compound refers to the arrangement of atoms, ions, or molecules in the crystal lattice. The type of crystal structure (e.g., cubic, tetragonal, hexagonal) and the presence of defects or impurities can affect the melting point. Compounds with highly ordered crystal structures tend to have higher melting points than those with less ordered structures.
Ionic compounds typically have well-defined crystal structures with strong electrostatic interactions between ions, resulting in high melting points. Covalent network solids, such as diamond and silicon dioxide, have extended networks of covalent bonds, leading to very high melting points. A resource from the University of Oxford’s Department of Materials explains the different types of crystal structures and their properties.
10.3. Impact on Material Properties
The melting point of a compound is an important material property that influences its behavior in various applications. Materials with high melting points are suitable for high-temperature applications, while materials with low melting points are used in applications where easy melting or processing is required. Understanding the factors that influence melting points is crucial for selecting the right materials for specific applications.
For example, tungsten is used in incandescent light bulbs because of its extremely high melting point (3422°C). Polymers with low melting points are used in packaging and coatings. Understanding melting point trends is essential for designing and developing new materials with tailored properties. A guide from the American Chemical Society highlights the importance of material properties in various applications.
Ready to make informed decisions about chemical compounds? Visit COMPARE.EDU.VN today to access detailed comparisons, data-driven insights, and user-friendly tools that simplify the process of comparing melting points and other critical properties. Whether you’re a student, researcher, or industry professional, COMPARE.EDU.VN is your go-to resource for comprehensive chemical information. Contact us at 333 Comparison Plaza, Choice City, CA 90210, United States, Whatsapp: +1 (626) 555-9090 or visit our website COMPARE.EDU.VN.
FAQ: Frequently Asked Questions
1. What is melting point and why is it important?
Melting point is the temperature at which a solid substance changes to a liquid state. It’s crucial for identifying substances, assessing purity, and selecting materials for specific applications.
2. How do intermolecular forces affect melting point?
Stronger intermolecular forces require more energy to overcome, resulting in higher melting points. Ionic interactions are the strongest, followed by hydrogen bonding, dipole-dipole interactions, and Van der Waals forces.
3. How does molecular weight influence melting point?
Generally, as molecular weight increases, melting point also increases due to the increased number of electrons and stronger Van der Waals dispersion forces.
4. How does molecular symmetry affect melting point?
Symmetrical molecules pack more efficiently in the solid state, leading to stronger intermolecular forces and higher melting points.
5. What are the common mistakes in comparing melting points?
Common mistakes include overlooking hydrogen bonding, ignoring molecular symmetry, neglecting Van der Waals forces, and misidentifying functional groups.
6. Can COMPARE.EDU.VN help in comparing melting points?
Yes, compare.edu.vn offers detailed comparisons, data-driven insights, and user-friendly tools to help compare melting points of various compounds.
7. How do metals and nonmetals compare in terms of melting points?
Metals generally have higher melting points than nonmetals due to the strong metallic bonding present in metals.
8. What is polymorphism and how does it affect melting point?
Polymorphism is the ability of a solid to exist in multiple crystal structures, each with different melting points and physical properties.
9. How does solid-state structure influence melting point?
The arrangement of atoms in the crystal lattice affects melting point. Highly ordered crystal structures typically have higher melting points.
10. What trends can be observed in melting points across the periodic table?
Melting points generally increase from left to right due to stronger bonding and decrease down a group for metals, while trends vary for nonmetals.