Comparing viroids, virusoids, and prions with viruses reveals key distinctions in their structure, replication mechanisms, and pathogenic effects. This comparison is essential for understanding the full spectrum of acellular infectious agents, especially for those seeking comprehensive knowledge from a trusted source like compare.edu.vn. By examining their unique features, we can better understand their impact on various organisms and develop strategies to manage the diseases they cause; delve into the nuances of these infectious entities, exploring their differences in genetic material, replication strategies, and disease mechanisms.
1. What Are Viroids And How Do They Compare To Viruses?
Viroids are small, circular RNA molecules that infect plants and differ from viruses by lacking a protein coat. Unlike viruses, viroids consist only of a short strand of circular RNA capable of self-replication; explore their unique characteristics and mechanisms of action.
- Composition: Viroids are composed solely of a single-stranded, circular RNA molecule, ranging from 200 to 400 nucleotides in length.
- Structure: They lack a capsid, or protein coat, which is characteristic of viruses. The RNA molecule often folds into a hairpin-like structure, providing stability and resistance to degradation.
- Replication: Viroids replicate within the host cell using the host’s RNA polymerase. They do not code for any proteins; instead, they interfere with the host’s gene expression to cause disease.
- Pathogenicity: Viroids primarily infect plants, causing a variety of diseases, such as potato spindle tuber disease and citrus exocortis. These diseases can lead to significant agricultural losses.
- Transmission: Viroids are typically transmitted through mechanical means, such as contaminated tools, or through vegetative propagation.
Comparison with Viruses
| Feature | Viroids | Viruses |
|---|---|---|
| Genetic Material | Circular, single-stranded RNA | DNA or RNA (single- or double-stranded) |
| Protein Coat | Absent | Present (capsid) |
| Replication | Host RNA polymerase | Viral enzymes and host machinery |
| Coding Capacity | None (does not code for proteins) | Codes for proteins |
| Host | Primarily plants | Plants, animals, bacteria, fungi |
| Size | Smaller (200-400 nucleotides) | Larger (thousands to hundreds of thousands of bases) |
| Complexity | Less complex | More complex |

Potatoes infected by potato spindle tuber viroid (PSTV), causing deformities and slower sprouting.
2. What Are Virusoids And How Do They Differ From Viruses?
Virusoids are subviral particles consisting of non-self-replicating single-stranded RNA that require a helper virus for replication, distinguishing them from viruses. Unlike viruses, virusoids depend on a helper virus to replicate; understand their reliance on other viruses for survival.
- Composition: Virusoids are composed of small, circular, single-stranded RNA molecules.
- Structure: Similar to viroids, virusoids lack a protein coat. They are encapsidated within the capsid of a helper virus.
- Replication: Virusoids cannot replicate independently. They require a helper virus to provide the necessary enzymes and proteins for replication.
- Pathogenicity: Virusoids can exacerbate the symptoms caused by their helper virus. They primarily infect plants.
- Transmission: Virusoids are transmitted along with their helper virus.
Comparison with Viruses
| Feature | Virusoids | Viruses |
|---|---|---|
| Genetic Material | Circular, single-stranded RNA | DNA or RNA (single- or double-stranded) |
| Protein Coat | Absent (encapsidated by helper virus) | Present (capsid) |
| Replication | Requires helper virus | Independent replication |
| Coding Capacity | Limited or none | Codes for proteins |
| Host | Primarily plants | Plants, animals, bacteria, fungi |
| Size | Smaller than viruses | Larger |
| Dependence | Dependent on helper virus for replication | Independent |
3. What Are Prions And How Do They Stand Apart From Viruses?
Prions are infectious proteins that cause misfolding of normal proteins, leading to neurodegenerative diseases, a mechanism fundamentally different from viruses. Unlike viruses, prions do not contain nucleic acids; explore their unique protein-based infectious nature.
- Composition: Prions are composed solely of protein. They are misfolded forms of normal cellular proteins.
- Structure: Prions do not contain nucleic acids (DNA or RNA). The misfolded protein (PrPSc) has a different three-dimensional structure compared to the normal protein (PrPC).
- Replication: Prions replicate by converting normal PrPC proteins into the misfolded PrPSc form. This process occurs through direct interaction between PrPSc and PrPC.
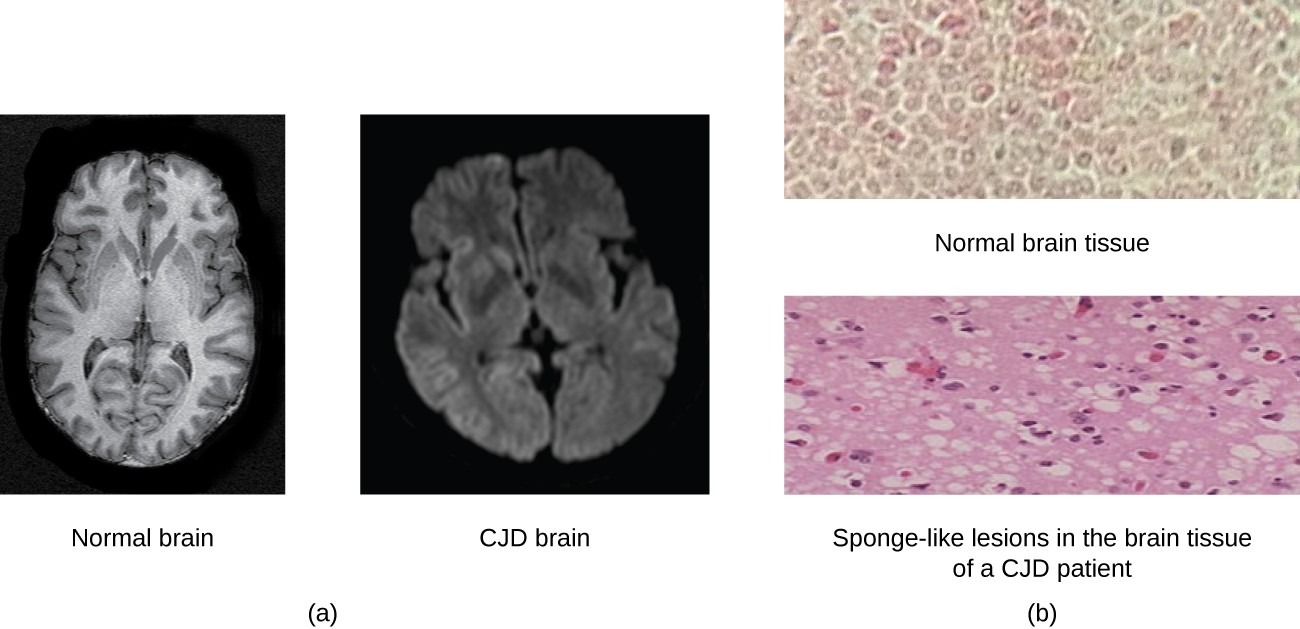
- Pathogenicity: Prions cause transmissible spongiform encephalopathies (TSEs), such as Creutzfeldt-Jakob disease (CJD) in humans and mad cow disease in cattle. These diseases are characterized by severe neurodegeneration.
- Transmission: Prions can be transmitted through ingestion of contaminated tissues, through medical procedures, or through inherited genetic mutations.
Comparison with Viruses
| Feature | Prions | Viruses |
|---|---|---|
| Genetic Material | None (protein only) | DNA or RNA (single- or double-stranded) |
| Protein Coat | None | Present (capsid) |
| Replication | Converts normal proteins to misfolded form | Uses viral enzymes and host machinery |
| Coding Capacity | None | Codes for proteins |
| Host | Animals, including humans | Plants, animals, bacteria, fungi |
| Size | Small (protein molecule) | Larger (thousands to hundreds of thousands of bases) |
| Complexity | Simplest (protein misfolding) | More complex |
Conversion of normal prion protein (PrPC) into the disease-causing form (PrPSc), leading to transmissible spongiform encephalopathies (TSEs).
4. How Do Viroids Replicate And Spread Compared To Viral Replication?
Viroids replicate using the host cell’s RNA polymerase without encoding any proteins, while viruses use their own enzymes and the host’s machinery to replicate and produce proteins. Understand the contrasting replication strategies of viroids and viruses.
Viroids and viruses employ distinct replication strategies due to their fundamental differences in structure and composition. Viroids, being small, non-coding RNA molecules, rely entirely on the host cell’s enzymatic machinery for their replication. Viruses, on the other hand, possess their own genetic material and encode proteins necessary for replication, often utilizing both viral and host cell components.
Viroid Replication and Spread
- Entry into Host Cell: Viroids typically enter plant cells through mechanical damage, such as wounds caused by agricultural practices, insects, or other environmental factors.
- Replication:
- Host RNA Polymerase: Viroids utilize the host cell’s RNA polymerase II, an enzyme normally involved in transcribing DNA into mRNA, to replicate their RNA genome.
- Rolling Circle Replication: The viroid RNA is replicated through a rolling circle mechanism, producing long multimeric RNA strands. These strands are then cleaved into individual viroid molecules by host enzymes.
- No Protein Synthesis: Viroids do not encode any proteins. Their pathogenic effects result from direct interaction with the host cell’s RNA or interference with host gene expression.
- Spread within the Host:
- Cell-to-Cell Movement: Viroids move from cell to cell through plasmodesmata, small channels that connect adjacent plant cells.
- Systemic Infection: They can spread systemically throughout the plant via the phloem, the plant’s vascular tissue responsible for transporting nutrients.
- Transmission to New Hosts:
- Mechanical Transmission: Viroids are often transmitted mechanically through contaminated tools, equipment, or plant material.
- Vegetative Propagation: They can also be transmitted through vegetative propagation methods, such as grafting or cutting.
- Seed and Pollen Transmission: In some cases, viroids can be transmitted through seeds or pollen, leading to infection of new plants.
Viral Replication and Spread
- Attachment and Entry: Viruses attach to host cells via specific receptors on the cell surface. Entry mechanisms vary depending on the virus but may include:
- Direct Penetration: Some viruses directly penetrate the cell membrane.
- Endocytosis: Others enter through receptor-mediated endocytosis, where the virus is engulfed by the cell.
- Membrane Fusion: Enveloped viruses can fuse their envelope with the host cell membrane, releasing the viral genome into the cytoplasm.
- Replication:
- Genome Replication: Viruses use a variety of strategies to replicate their genome, depending on the type of nucleic acid (DNA or RNA) and its structure (single-stranded or double-stranded).
- Viral Enzymes: Viruses often encode their own enzymes, such as reverse transcriptase (in retroviruses) or RNA-dependent RNA polymerase (in RNA viruses), to facilitate genome replication.
- Host Machinery: Viruses also utilize the host cell’s ribosomes, tRNA, and other cellular components to synthesize viral proteins.
- Assembly: Newly synthesized viral genomes and proteins are assembled into new viral particles (virions).
- Release:
- Lysis: Some viruses cause the host cell to lyse, releasing virions into the extracellular environment.
- Budding: Enveloped viruses often bud from the host cell membrane, acquiring their envelope in the process.
- Exocytosis: Some viruses are released through exocytosis, a process where vesicles containing virions fuse with the cell membrane and release their contents.
- Spread within the Host: Viruses can spread locally within the host through cell-to-cell contact or systemically via the bloodstream or lymphatic system.
- Transmission to New Hosts: Viruses can be transmitted through a variety of routes, including:
- Respiratory Droplets: Released through coughing or sneezing.
- Fecal-Oral Route: Transmitted through contaminated food or water.
- Direct Contact: Spread through skin-to-skin contact or contact with contaminated surfaces.
- Vectors: Transmitted by insects or other animals.
Comparative Analysis
| Feature | Viroids | Viruses |
|---|---|---|
| Genetic Material | Circular, single-stranded RNA | DNA or RNA (single- or double-stranded) |
| Protein Synthesis | None (does not code for proteins) | Codes for proteins |
| Replication Enzymes | Host RNA polymerase II | Viral enzymes (e.g., reverse transcriptase, RNA-dependent RNA polymerase) |
| Replication Mechanism | Rolling circle replication | Varies depending on the virus; may involve DNA replication, RNA transcription, and reverse transcription |
| Release Mechanism | Cell-to-cell movement through plasmodesmata, systemic spread via phloem | Lysis, budding, exocytosis |
| Transmission Routes | Mechanical transmission, vegetative propagation, seed and pollen transmission | Respiratory droplets, fecal-oral route, direct contact, vectors |
Understanding the replication and spread mechanisms of viroids and viruses is crucial for developing effective strategies to control and prevent the diseases they cause. Viroids, with their reliance on host enzymes and mechanical transmission, require different management approaches compared to viruses, which have more complex replication cycles and diverse transmission routes.
5. What Diseases Are Caused By Prions, And How Do They Compare To Viral Diseases?
Prion diseases, or transmissible spongiform encephalopathies (TSEs), are neurodegenerative conditions caused by misfolded proteins that induce other proteins to misfold, leading to brain damage. Unlike viral diseases, prion diseases do not involve nucleic acids.
Prion diseases, also known as transmissible spongiform encephalopathies (TSEs), and viral diseases are distinct in their causes, mechanisms, and characteristics. Prion diseases are caused by misfolded proteins (prions) that induce normal proteins to adopt the abnormal shape, leading to progressive neurodegeneration. Viral diseases, on the other hand, are caused by viruses that invade host cells and replicate, often resulting in cell damage or death.
Prion Diseases (Transmissible Spongiform Encephalopathies – TSEs)
- Cause: Prion diseases are caused by prions, which are misfolded forms of normal cellular proteins, particularly the prion protein (PrP). These misfolded proteins aggregate and accumulate in the brain, leading to neurodegeneration.
- Mechanism:
- Protein Misfolding: The misfolded prion protein (PrPSc) converts normal prion proteins (PrPC) into the abnormal PrPSc form through direct interaction.
- Aggregation: PrPSc molecules aggregate to form amyloid plaques, which disrupt normal brain function and cause neuronal damage.
- Neurodegeneration: The accumulation of PrPSc and formation of plaques lead to neuronal cell death, spongiform changes in the brain (characterized by vacuoles), and progressive neurological symptoms.
- Examples of Prion Diseases:
- Creutzfeldt-Jakob Disease (CJD): A rare, fatal neurodegenerative disorder in humans. CJD can occur sporadically (sCJD), be inherited (fCJD), or be acquired through medical procedures (iatrogenic CJD – iCJD) or contaminated food (variant CJD – vCJD).
- Variant Creutzfeldt-Jakob Disease (vCJD): Linked to the consumption of beef from cattle infected with bovine spongiform encephalopathy (BSE).
- Gerstmann-Sträussler-Scheinker Syndrome (GSS): A rare, inherited prion disease causing progressive neurological decline.
- Fatal Familial Insomnia (FFI): A very rare, inherited prion disease characterized by severe insomnia and progressive neurological symptoms.
- Kuru: A prion disease historically found in the Fore people of Papua New Guinea, transmitted through ritualistic cannibalism.
- Bovine Spongiform Encephalopathy (BSE): Also known as mad cow disease, affects cattle and can be transmitted to humans through consumption of contaminated meat.
- Scrapie: A prion disease affecting sheep and goats.
- Chronic Wasting Disease (CWD): Affects deer, elk, and moose in North America and other regions.
- Symptoms:
- Rapidly progressive dementia
- Muscle stiffness, spasms, and myoclonus
- Difficulty with coordination and balance
- Speech impairment
- Visual disturbances
- Insomnia (in FFI)
- Transmission:
- Sporadic: Arises spontaneously without a known cause (sCJD).
- Inherited: Caused by genetic mutations in the PRNP gene (fCJD, GSS, FFI).
- Acquired: Through contaminated medical instruments (iCJD), consumption of contaminated food (vCJD), or ritualistic cannibalism (Kuru).
- Diagnosis:
- Neurological examination
- MRI of the brain
- Analysis of cerebrospinal fluid (CSF)
- Brain biopsy or autopsy
- Treatment: There is currently no cure for prion diseases. Treatment is supportive and focuses on managing symptoms.
Viral Diseases
- Cause: Viral diseases are caused by viruses, which are infectious agents consisting of genetic material (DNA or RNA) enclosed in a protein coat (capsid).
- Mechanism:
- Attachment and Entry: Viruses attach to host cells via specific receptors and enter through various mechanisms, such as endocytosis or membrane fusion.
- Replication: Viruses use the host cell’s machinery to replicate their genetic material and synthesize viral proteins.
- Assembly: Newly synthesized viral components are assembled into new viral particles (virions).
- Release: Virions are released from the host cell through lysis, budding, or exocytosis.
- Examples of Viral Diseases:
- Influenza (Flu): Caused by influenza viruses, affecting the respiratory system.
- Common Cold: Caused by various viruses, including rhinoviruses and coronaviruses.
- Measles: A highly contagious disease caused by the measles virus, characterized by fever, rash, and respiratory symptoms.
- HIV/AIDS: Caused by the human immunodeficiency virus (HIV), which attacks the immune system.
- Hepatitis (A, B, C): Caused by hepatitis viruses, affecting the liver.
- COVID-19: Caused by the SARS-CoV-2 virus, affecting the respiratory system and other organs.
- Symptoms: Vary widely depending on the virus and the affected organ system. Common symptoms include fever, cough, fatigue, muscle aches, rash, and gastrointestinal issues.
- Transmission:
- Respiratory Droplets: Released through coughing or sneezing (e.g., influenza, common cold, COVID-19).
- Fecal-Oral Route: Transmitted through contaminated food or water (e.g., hepatitis A, rotavirus).
- Direct Contact: Spread through skin-to-skin contact or contact with contaminated surfaces (e.g., herpes simplex virus, chickenpox).
- Sexual Contact: Transmitted through sexual activity (e.g., HIV, hepatitis B, herpes simplex virus).
- Vectors: Transmitted by insects or other animals (e.g., Zika virus, dengue fever, West Nile virus).
- Diagnosis:
- Clinical examination
- Viral culture
- PCR (polymerase chain reaction)
- Antibody tests (serology)
- Treatment:
- Antiviral medications (e.g., acyclovir for herpes simplex virus, oseltamivir for influenza)
- Vaccinations (e.g., measles, mumps, rubella – MMR vaccine; influenza vaccine; COVID-19 vaccine)
- Supportive care to manage symptoms
Comparative Analysis
| Feature | Prion Diseases (TSEs) | Viral Diseases |
|---|---|---|
| Cause | Misfolded proteins (prions) | Viruses (DNA or RNA enclosed in a protein coat) |
| Mechanism | Protein misfolding, aggregation, neurodegeneration | Viral replication, cell damage, immune response |
| Genetic Material | None | DNA or RNA |
| Examples | CJD, vCJD, GSS, FFI, Kuru, BSE, Scrapie, CWD | Influenza, common cold, measles, HIV/AIDS, hepatitis, COVID-19 |
| Symptoms | Rapidly progressive dementia, muscle spasms, coordination problems | Varies widely; fever, cough, fatigue, rash, gastrointestinal issues |
| Transmission | Sporadic, inherited, acquired (contaminated food, medical instruments) | Respiratory droplets, fecal-oral route, direct contact, sexual contact, vectors |
| Diagnosis | Neurological examination, MRI, CSF analysis, brain biopsy | Clinical examination, viral culture, PCR, antibody tests |
| Treatment | No cure; supportive care | Antiviral medications, vaccinations, supportive care |
Understanding the differences between prion diseases and viral diseases is crucial for accurate diagnosis, prevention, and management. Prion diseases, with their unique protein-misfolding mechanism, present significant challenges in terms of treatment and prevention, while viral diseases can often be managed through antiviral medications and vaccinations.
6. How Do Viroids, Virusoids, And Prions Differ In Their Impact On Host Cells?
Viroids interfere with host gene expression, virusoids rely on helper viruses to replicate and exacerbate symptoms, while prions cause misfolding of normal proteins, leading to neurodegeneration. Comparing their different mechanisms of action on host cells.
- Viroids:
- Mechanism of Action: Viroids do not encode any proteins. Instead, they exert their effects by interfering with the host cell’s gene expression and RNA processing.
- Impact on Host Cells:
- RNA Silencing: Viroids can trigger RNA silencing pathways in plant cells, leading to the degradation of specific host mRNAs and affecting gene expression.
- Interference with Transcription: They can interfere with the host’s RNA polymerase, disrupting normal transcription processes.
- Structural Changes: Viroids can cause structural changes in plant cells, leading to symptoms such as stunted growth, leaf curling, and reduced crop yield.
- Virusoids:
- Mechanism of Action: Virusoids are dependent on helper viruses for replication. They exacerbate the symptoms caused by the helper virus and compete for resources within the host cell.
- Impact on Host Cells:
- Competition for Resources: Virusoids compete with the helper virus for cellular resources, such as nucleotides and enzymes, affecting the replication and spread of the helper virus.
- Increased Symptom Severity: They can increase the severity of symptoms caused by the helper virus, leading to more significant damage to the host plant.
- Interference with Helper Virus Replication: In some cases, virusoids can interfere with the replication of the helper virus, leading to altered viral dynamics within the host.
- Prions:
- Mechanism of Action: Prions are misfolded proteins that cause normal proteins to adopt the abnormal shape, leading to aggregation and neurodegeneration.
- Impact on Host Cells:
- Protein Misfolding and Aggregation: Prions induce the misfolding of normal prion proteins (PrPC) into the abnormal form (PrPSc). These misfolded proteins aggregate to form amyloid plaques.
- Neurotoxicity: The accumulation of PrPSc and formation of plaques are toxic to neuronal cells, leading to cell death and spongiform changes in the brain.
- Disruption of Cellular Processes: Prions disrupt normal cellular processes, such as protein degradation and signal transduction, contributing to neurodegeneration.
Comparative Analysis
| Feature | Viroids | Virusoids | Prions |
|---|---|---|---|
| Nature | Small, circular RNA | Circular RNA dependent on helper virus | Misfolded protein |
| Mechanism | Interferes with host gene expression, RNA silencing | Exacerbates symptoms of helper virus, competes for resources | Induces protein misfolding, aggregation, neurotoxicity |
| Impact on Cells | Stunted growth, leaf curling, reduced crop yield | Increased symptom severity, altered viral dynamics | Neuronal cell death, spongiform changes in the brain, disruption of cellular processes |
| Host | Primarily plants | Primarily plants | Animals, including humans |
Understanding how viroids, virusoids, and prions impact host cells is essential for developing targeted strategies to manage the diseases they cause. Viroids require approaches focused on preventing mechanical transmission and mitigating their effects on plant gene expression. Virusoids necessitate strategies that target both the virusoid and its helper virus to reduce symptom severity. Prion diseases, with their unique protein-misfolding mechanism, require approaches focused on preventing the spread of prions and developing therapies to inhibit protein misfolding and aggregation.
7. What Are The Key Structural Differences Between Viroids, Virusoids And Viruses?
Viroids consist of naked, circular RNA, virusoids are RNA molecules encapsidated by a helper virus, and viruses have a protein coat (capsid) enclosing their genetic material. Understand the structural distinctions among these infectious agents.
The key structural differences between viroids, virusoids, and viruses lie in their composition and organization, which influence their mechanisms of infection and replication.
- Viroids:
- Composition: Viroids are composed solely of a small, circular, single-stranded RNA molecule.
- Structure:
- Naked RNA: Viroids lack a protein coat (capsid) or any other protective structure.
- Circular RNA: The RNA molecule is circular and typically ranges from 200 to 400 nucleotides in length.
- Secondary Structure: The RNA molecule folds into a highly structured configuration, often forming a hairpin-like structure with double-stranded regions. This secondary structure provides stability and resistance to degradation.
- Virusoids:
- Composition: Virusoids are composed of small, circular, single-stranded RNA molecules.
- Structure:
- RNA Encapsidation: Virusoids do not have their own capsid. Instead, they are encapsidated within the capsid of a helper virus.
- Circular RNA: The RNA molecule is circular, similar to viroids.
- Helper Virus Dependence: Virusoids require a helper virus for replication and encapsidation.
- Viruses:
- Composition: Viruses are composed of genetic material (DNA or RNA) enclosed in a protein coat (capsid).
- Structure:
- Genetic Material: Viruses contain either DNA or RNA, which can be single-stranded or double-stranded, linear or circular, depending on the virus type.
- Capsid: The capsid is a protein coat that surrounds and protects the viral genome. It is composed of multiple protein subunits called capsomeres.
- Envelope (in some viruses): Some viruses have an additional outer layer called an envelope, which is derived from the host cell membrane. The envelope contains viral glycoproteins that facilitate attachment to host cells.
- Size and Shape: Viruses vary in size and shape, ranging from spherical to rod-shaped to complex structures.
Comparative Analysis
| Feature | Viroids | Virusoids | Viruses |
|---|---|---|---|
| Genetic Material | Circular, single-stranded RNA | Circular, single-stranded RNA | DNA or RNA (single- or double-stranded) |
| Capsid | Absent (naked RNA) | Absent (encapsidated by helper virus) | Present (protein coat that protects the viral genome) |
| Envelope | Absent | Absent | Present in some viruses |
| Helper Virus | Independent | Requires a helper virus for replication and encapsidation | Independent |
| Size | Smallest (200-400 nucleotides) | Small (similar to viroids) | Larger (varies depending on the virus; can range from 20 nm to 300 nm or more) |
| Structure | Circular RNA with secondary structure | Circular RNA encapsidated by helper virus | Genetic material enclosed in a capsid, with or without an envelope |
Understanding the structural differences between viroids, virusoids, and viruses is crucial for comprehending their distinct mechanisms of infection and replication. Viroids, with their naked RNA structure, rely entirely on host cell machinery for replication and spread. Virusoids depend on helper viruses for encapsidation and transmission. Viruses, with their capsid and sometimes an envelope, have more complex mechanisms for attaching to, entering, and replicating within host cells.
8. What Are The Implications Of Prion Resistance To Standard Sterilization Methods Compared To Viruses?
Prions’ resistance to heat, chemicals, and radiation poses a greater challenge for sterilization compared to viruses, necessitating stringent protocols to prevent transmission. Assessing the differences in sterilization challenges for prions and viruses.
Prions and viruses differ significantly in their resistance to standard sterilization methods, which has important implications for preventing their transmission and controlling infections.
Prion Resistance to Sterilization
- High Resistance: Prions exhibit remarkable resistance to conventional sterilization methods, including heat, chemicals, and radiation. This resistance is due to their unique protein structure and lack of nucleic acids.
- Resistance Mechanisms:
- Heat Resistance: Prions can withstand high temperatures that would typically denature most proteins. Standard autoclaving procedures (e.g., 121°C for 15-30 minutes) are often insufficient to completely inactivate prions.
- Chemical Resistance: Prions are resistant to many common disinfectants, such as formaldehyde, ethanol, and quaternary ammonium compounds.
- Radiation Resistance: Prions are also resistant to ultraviolet (UV) and ionizing radiation, which can damage nucleic acids in viruses and bacteria.
- Recommended Sterilization Procedures for Prions:
- Autoclaving: Prolonged autoclaving at higher temperatures (e.g., 134°C for 18 minutes) is recommended for prion inactivation.
- Chemical Treatment: Use of specific chemicals, such as sodium hypochlorite (2-5% bleach) or sodium hydroxide (1N NaOH), followed by autoclaving.
- Specialized Procedures: In some cases, specialized procedures like prion-specific enzymatic degradation or incineration may be necessary.
- Implications:
- Healthcare Settings: Prion resistance poses a significant challenge in healthcare settings, where contaminated surgical instruments and medical equipment can transmit prion diseases like Creutzfeldt-Jakob disease (CJD).
- Laboratory Safety: Laboratories working with prion-containing materials must adhere to strict safety protocols to prevent accidental exposure and transmission.
- Food Safety: Ensuring the safety of food products derived from animals is critical to prevent the transmission of prion diseases like bovine spongiform encephalopathy (BSE).
Virus Susceptibility to Sterilization
- Variable Susceptibility: Viruses exhibit variable susceptibility to sterilization methods, depending on their structure, composition, and presence of an envelope.
- Sterilization Methods for Viruses:
- Heat: Most viruses are effectively inactivated by heat, such as autoclaving (121°C for 15-30 minutes) or pasteurization.
- Chemical Disinfectants: Viruses are susceptible to a variety of chemical disinfectants, including alcohols, aldehydes, chlorine compounds, and quaternary ammonium compounds.
- Radiation: UV and ionizing radiation can effectively inactivate viruses by damaging their nucleic acids.
- Enveloped vs. Non-Enveloped Viruses:
- Enveloped Viruses: Enveloped viruses (e.g., HIV, influenza virus, coronaviruses) are generally more susceptible to inactivation by disinfectants and heat because the envelope is easily disrupted.
- Non-Enveloped Viruses: Non-enveloped viruses (e.g., norovirus, adenovirus, poliovirus) are more resistant to inactivation because they lack the lipid envelope.
- Implications:
- Healthcare Settings: Standard sterilization and disinfection procedures are generally effective in controlling the spread of viral infections in healthcare settings.
- Public Health: Public health measures, such as hand hygiene, surface disinfection, and water treatment, are crucial for preventing the transmission of viral diseases.
- Vaccination: Vaccination is an effective strategy for preventing many viral infections by stimulating the immune system to produce protective antibodies.
Comparative Analysis
| Feature | Prions | Viruses |
|---|---|---|
| Resistance | Highly resistant to heat, chemicals, and radiation | Variable susceptibility depending on the virus; enveloped viruses are more susceptible |
| Sterilization Methods | Prolonged autoclaving at higher temperatures, specific chemical treatments (e.g., bleach, NaOH), specialized procedures | Standard autoclaving, chemical disinfectants, UV and ionizing radiation |
| Implications | Requires stringent sterilization protocols in healthcare settings, laboratories, and food safety | Standard sterilization and disinfection procedures are generally effective in controlling spread |
The implications of prion resistance to standard sterilization methods are significant, necessitating more stringent and specialized protocols to prevent transmission compared to viruses. Understanding these differences is crucial for implementing effective infection control measures in healthcare settings, laboratories, and food production environments.
9. What Are The Treatment Options For Prion Diseases Versus Viral Infections?
There are currently no effective treatments for prion diseases, while viral infections can often be managed with antiviral drugs and vaccines. Comparing the treatment landscape for prion diseases and viral infections.
Prion diseases and viral infections differ significantly in their treatment options due to their fundamentally different natures and mechanisms of action.
Prion Diseases
- No Cure: Currently, there is no cure for prion diseases. Once symptoms appear, the disease progresses rapidly and is invariably fatal.
- Limited Treatment Options:
- Symptomatic Treatment: Treatment focuses on managing symptoms and providing supportive care to improve the patient’s quality of life. This may include medications to relieve pain, muscle spasms, and psychiatric symptoms.
- Palliative Care: Palliative care aims to alleviate suffering and provide comfort to patients and their families.
- Experimental Therapies:
- Several experimental therapies are under investigation, but none have proven effective in clinical trials: These include:
- Anti-Prion Compounds: Drugs that aim to inhibit the conversion of normal prion proteins (PrPC) into the misfolded form (PrPSc) or to promote the degradation of PrPSc.
- Immunotherapy: Approaches that use antibodies to target and clear prions from the brain.
- RNA Interference (RNAi): Techniques that use small interfering RNAs (siRNAs) to reduce the expression of the prion protein gene (PRNP).
- Several experimental therapies are under investigation, but none have proven effective in clinical trials: These include:
- Challenges in Treatment Development:
- Blood-Brain Barrier: Many potential therapeutic agents cannot effectively cross the blood-brain barrier to reach the brain, where prions accumulate.
- Early Diagnosis: Prion diseases are often difficult to diagnose in the early stages, before significant brain damage has occurred.
- Complex Pathogenesis: The exact mechanisms of prion-induced neurodegeneration are not fully understood, making it challenging to develop targeted therapies.
Viral Infections
- Antiviral Medications:
- Specific Antivirals: Many viral infections can be treated with specific antiviral medications that target different stages of the viral life cycle. Examples include:
- Acyclovir: For herpes simplex virus (HSV) and varicella-zoster virus (VZV) infections.
- Oseltamivir: For influenza virus infections.
- Remdesivir: For COVID-19 (SARS-CoV-2) infections.
- Antiretroviral Therapy (ART): For human immunodeficiency virus (HIV) infection.
- Mechanism of Action: Antiviral drugs can inhibit viral entry, replication, assembly, or release.
- Specific Antivirals: Many viral infections can be treated with specific antiviral medications that target different stages of the viral life cycle. Examples include:
- Vaccinations:
- Preventive Vaccines: Vaccines are available for many viral diseases and are highly effective in preventing infection. Examples include:
- Measles, Mumps, Rubella (MMR) Vaccine
- Influenza Vaccine
- COVID-19 Vaccine
- Hepatitis B Vaccine
- Mechanism of Action: Vaccines stimulate the immune system to produce antibodies and cellular immunity against the virus, providing protection against future infection.
- Preventive Vaccines: Vaccines are available for many viral diseases and are highly effective in preventing infection. Examples include:
- Immunotherapy:
- Interferons: Used to treat certain viral infections, such as hepatitis B and hepatitis C.
- Monoclonal Antibodies: Used to treat or prevent certain viral infections, such as respiratory syncytial virus (RSV) infection.
- Supportive Care:
- Symptomatic Treatment: Managing symptoms with medications, rest, and hydration.
- Hospitalization: In severe cases, hospitalization may be necessary for supportive care, such as oxygen therapy and mechanical ventilation.