Comparing boiling points involves understanding the intermolecular forces at play. At COMPARE.EDU.VN, we break down the science, making it easy to predict and compare boiling points based on molecular structure and bonding. Master the principles of intermolecular forces, molecular weight influence, and symmetry effects, and you’ll be able to accurately estimate and compare boiling temperatures, phase transition temperatures, and vaporization points.
Table of Contents
- Understanding Boiling Point: The Basics
- Intermolecular Forces: The Key to Comparing Boiling Points
- 2.1 Ionic Interactions: The Strongest Force
- 2.2 Hydrogen Bonding: A Potent Attraction
- 2.3 Dipole-Dipole Interactions: Moderate Strength
- 2.4 Van der Waals Dispersion Forces: The Weakest Link
- Trend #1: Intermolecular Forces and Boiling Point
- 3.1 Case Study: Butane Derivatives
- 3.2 Amine and Carboxylic Acid Isomers
- Trend #2: Molecular Weight and Boiling Point
- 4.1 The Role of Van der Waals Forces
- 4.2 Surface Area and Molecular Interactions
- Trend #3: Symmetry and Boiling Point
- 5.1 Molecular Shape Matters
- 5.2 The Impact on Hydrogen Bonding
- Factors Affecting Boiling Point: A Detailed Look
- 6.1 Polarity of Molecules
- 6.2 Molecular Size and Shape
- 6.3 Presence of Functional Groups
- Boiling Point Trends in Homologous Series
- 7.1 Alkanes: A Simple Example
- 7.2 Alcohols, Ethers, and Carboxylic Acids
- How to Predict Boiling Points: A Step-by-Step Guide
- 8.1 Identify Intermolecular Forces
- 8.2 Consider Molecular Weight
- 8.3 Evaluate Molecular Symmetry
- Boiling Point vs. Melting Point: What’s the Difference?
- 9.1 Boiling Point: Vaporization Dynamics
- 9.2 Melting Point: Solid-State Transitions
- Examples of Boiling Point Comparisons
- 10.1 Comparing Isomers
- 10.2 Comparing Compounds with Different Functional Groups
- The Significance of Boiling Point in Various Fields
- 11.1 Chemistry and Chemical Engineering
- 11.2 Pharmaceuticals and Drug Development
- 11.3 Food Science
- Tools and Resources for Comparing Boiling Points
- 12.1 Online Databases
- 12.2 Software and Apps
- Advanced Topics in Boiling Point
- 13.1 Azeotropes
- 13.2 Vacuum Distillation
- Common Mistakes to Avoid When Comparing Boiling Points
- 14.1 Overlooking Intermolecular Forces
- 14.2 Ignoring Molecular Shape
- Real-World Applications of Boiling Point Knowledge
- 15.1 Distillation Processes
- 15.2 Solvent Selection
- Expert Insights on Comparing Boiling Points
- 16.1 Tips from Experienced Chemists
- 16.2 Case Studies
- Future Trends in Boiling Point Research
- 17.1 Nanomaterials
- 17.2 Green Chemistry
- Practical Tips for Students and Professionals
- 18.1 Study Techniques
- 18.2 Professional Development
- Frequently Asked Questions (FAQs) About Boiling Points
- Conclusion: Mastering the Art of Boiling Point Comparison with COMPARE.EDU.VN
1. Understanding Boiling Point: The Basics
Boiling point is the temperature at which a liquid changes into a gas. This transition occurs when the vapor pressure of the liquid equals the surrounding atmospheric pressure. To effectively compare boiling points, one must understand that it’s essentially a measure of the energy required to overcome the intermolecular forces holding the molecules together in the liquid state. Higher boiling points indicate stronger intermolecular attractions. This involves looking at intermolecular forces, molecular weight, and molecular symmetry. These aspects dictate how molecules interact, influence their thermal stability, and determine at what temperature they transition to a gaseous state.
2. Intermolecular Forces: The Key to Comparing Boiling Points
Intermolecular forces (IMFs) are the attractive or repulsive forces that mediate interaction between molecules, including London dispersion forces, dipole-dipole interactions, hydrogen bonding, and ionic interactions. Understanding these forces is crucial for comparing boiling points. The stronger the intermolecular forces, the more energy (and thus higher temperature) is required to separate the molecules and cause boiling.
- Ionic: Very strong forces of attraction between oppositely charged ions.
- Hydrogen bonding: Attraction between hydrogen atom bonded to a highly electronegative atom (O, N, F) and another electronegative atom.
- Dipole-dipole: Attraction between polar molecules.
- Van der Waals dispersion forces: Weak, temporary attractions between all molecules.
2.1 Ionic Interactions: The Strongest Force
Ionic interactions are the strongest intermolecular forces. They occur between ions of opposite charges. Compounds with ionic bonds generally have very high boiling points because a significant amount of energy is required to break these strong electrostatic attractions. Sodium chloride (NaCl), commonly known as table salt, is a classic example, with an extremely high melting and boiling point due to the robust ionic lattice structure.
2.2 Hydrogen Bonding: A Potent Attraction
Hydrogen bonding is a strong type of dipole-dipole interaction that occurs when a hydrogen atom is bonded to a highly electronegative atom such as oxygen (O), nitrogen (N), or fluorine (F). These bonds are particularly strong and lead to significantly higher boiling points in compounds where they are present. Water (H2O), with its extensive hydrogen bonding network, has a much higher boiling point than other molecules of similar molecular weight that lack hydrogen bonds.
2.3 Dipole-Dipole Interactions: Moderate Strength
Dipole-dipole interactions occur between polar molecules, which have a positive end and a negative end due to uneven electron distribution. These forces are stronger than Van der Waals forces but weaker than hydrogen bonds. For example, acetone has a higher boiling point than nonpolar molecules like pentane because of the dipole-dipole interactions between acetone molecules.
2.4 Van der Waals Dispersion Forces: The Weakest Link
Van der Waals dispersion forces, also known as London dispersion forces, are the weakest type of intermolecular force. They occur between all molecules, polar or nonpolar, and are caused by temporary fluctuations in electron distribution, creating temporary dipoles. These forces become more significant as the size and surface area of the molecule increase. For instance, larger alkanes have higher boiling points than smaller alkanes due to increased Van der Waals forces.
3. Trend #1: Intermolecular Forces and Boiling Point
The strength of intermolecular forces directly affects the boiling point of a substance. Substances with stronger IMFs require more energy to overcome these attractions, resulting in higher boiling points.
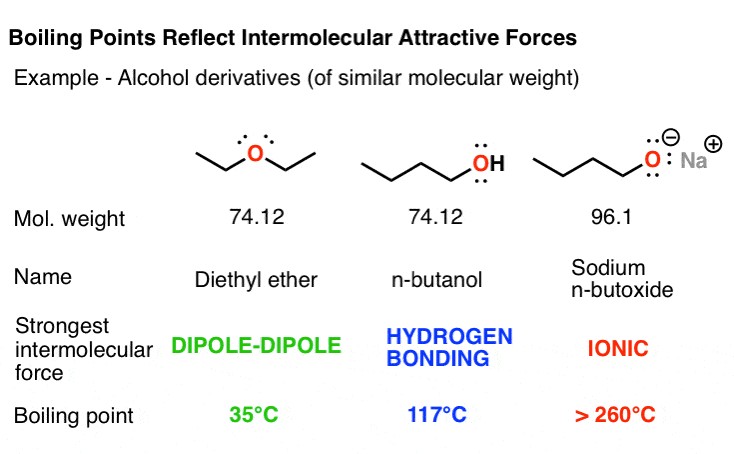
3.1 Case Study: Butane Derivatives
Let’s compare butane derivatives to illustrate the impact of intermolecular forces on boiling points:
- Diethyl ether (C4H10O): Held together by dipole-dipole interactions due to polarized C-O bonds, with a boiling point of 35°C.
- 1-Butanol (C4H10O): Contains a hydroxyl group capable of hydrogen bonding, resulting in a significantly higher boiling point of 117°C compared to diethyl ether.
- Sodium butoxide (C4H9ONa): An ionic compound that melts at a very high temperature (above 260°C) and decomposes before boiling, illustrating the strength of ionic interactions.
- Butane (C4H10): Experiences only weak Van der Waals dispersion forces, leading to a low boiling point of 0°C.
3.2 Amine and Carboxylic Acid Isomers
Similarly, consider amine and carboxylic acid isomers:
- Amines can form hydrogen bonds, but less strongly than alcohols, resulting in intermediate boiling points.
- Carboxylic acids form strong hydrogen bonds due to the presence of both a carbonyl and a hydroxyl group, leading to higher boiling points.
4. Trend #2: Molecular Weight and Boiling Point
For molecules with similar functional groups and intermolecular forces, boiling point generally increases with molecular weight. Larger molecules have more electrons, leading to stronger Van der Waals dispersion forces.
4.1 The Role of Van der Waals Forces
Van der Waals forces are proportional to the surface area of the molecule. As molecular weight increases, so does the surface area, leading to stronger intermolecular attractions and higher boiling points.
4.2 Surface Area and Molecular Interactions
Longer molecules have more surface area, allowing for greater contact and stronger Van der Waals interactions. This is why longer-chain alkanes have higher boiling points than shorter-chain alkanes.
5. Trend #3: Symmetry and Boiling Point
Molecular symmetry affects how well molecules can pack together. More symmetrical molecules tend to have lower boiling points compared to their less symmetrical counterparts with similar molecular weights and intermolecular forces.
5.1 Molecular Shape Matters
Rod-like molecules can align more closely than spherical molecules, leading to stronger intermolecular forces and higher boiling points.
5.2 The Impact on Hydrogen Bonding
The position of functional groups, such as hydroxyl groups in alcohols, affects their ability to form hydrogen bonds. More exposed functional groups lead to stronger hydrogen bonding and higher boiling points.
6. Factors Affecting Boiling Point: A Detailed Look
Several factors influence the boiling point of a substance, including the polarity of molecules, their size and shape, and the presence of specific functional groups.
6.1 Polarity of Molecules
Polar molecules have a positive and negative end, resulting in dipole-dipole interactions that increase the boiling point. Nonpolar molecules only exhibit weak Van der Waals forces, leading to lower boiling points.
6.2 Molecular Size and Shape
Larger molecules have more electrons, leading to stronger Van der Waals forces and higher boiling points. Molecular shape also affects how closely molecules can pack together, influencing the strength of intermolecular forces.
6.3 Presence of Functional Groups
Functional groups such as hydroxyl (-OH), carbonyl (C=O), and amine (-NH2) groups introduce dipole-dipole interactions or hydrogen bonding, significantly increasing the boiling point.
7. Boiling Point Trends in Homologous Series
Homologous series are sequences of organic compounds with the same functional group and similar chemical properties, where each member differs by a CH2 group. Analyzing these series provides insights into boiling point trends.
7.1 Alkanes: A Simple Example
In alkanes, boiling point increases with chain length due to increased Van der Waals forces. For example, methane (CH4) has a much lower boiling point than octane (C8H18).
7.2 Alcohols, Ethers, and Carboxylic Acids
Alcohols exhibit higher boiling points than alkanes of similar molecular weight due to hydrogen bonding. Carboxylic acids, with their ability to form strong hydrogen bonds, have even higher boiling points. Ethers, which can only form dipole-dipole interactions, have intermediate boiling points.
8. How to Predict Boiling Points: A Step-by-Step Guide
Predicting boiling points involves a systematic approach, considering the various factors influencing intermolecular forces.
8.1 Identify Intermolecular Forces
The first step is to identify the types of intermolecular forces present in the compound:
- Ionic interactions for ionic compounds.
- Hydrogen bonding for compounds with O-H, N-H, or F-H bonds.
- Dipole-dipole interactions for polar molecules.
- Van der Waals dispersion forces for all molecules.
8.2 Consider Molecular Weight
For molecules with similar intermolecular forces, consider the molecular weight. Larger molecules generally have higher boiling points due to increased Van der Waals forces.
8.3 Evaluate Molecular Symmetry
Assess the molecular symmetry. More symmetrical molecules tend to have lower boiling points compared to their less symmetrical counterparts.
9. Boiling Point vs. Melting Point: What’s the Difference?
Boiling point and melting point are both phase transition temperatures, but they refer to different processes: boiling involves the transition from liquid to gas, while melting involves the transition from solid to liquid.
9.1 Boiling Point: Vaporization Dynamics
Boiling point is the temperature at which the vapor pressure of a liquid equals the surrounding atmospheric pressure, leading to rapid vaporization.
9.2 Melting Point: Solid-State Transitions
Melting point is the temperature at which a solid transitions to a liquid state, indicating the strength of intermolecular forces in the solid lattice.
10. Examples of Boiling Point Comparisons
Comparing boiling points of different compounds highlights the importance of intermolecular forces, molecular weight, and molecular symmetry.
10.1 Comparing Isomers
Isomers are molecules with the same molecular formula but different structures. Comparing their boiling points reveals the impact of molecular shape and functional group position on intermolecular forces.
10.2 Comparing Compounds with Different Functional Groups
Comparing compounds with different functional groups demonstrates the effect of various intermolecular forces on boiling points. For example, comparing ethanol (alcohol) and dimethyl ether (ether) shows the impact of hydrogen bonding on boiling point.
11. The Significance of Boiling Point in Various Fields
Boiling point is a critical property in various fields, including chemistry, pharmaceuticals, and food science.
11.1 Chemistry and Chemical Engineering
In chemistry and chemical engineering, boiling point is essential for distillation, separation, and purification processes.
11.2 Pharmaceuticals and Drug Development
In pharmaceuticals, boiling point is used to characterize and purify drug compounds, ensuring their stability and efficacy.
11.3 Food Science
In food science, boiling point affects cooking temperatures, flavor development, and the preservation of food products.
12. Tools and Resources for Comparing Boiling Points
Several tools and resources are available for comparing and predicting boiling points.
12.1 Online Databases
Online databases such as the National Institute of Standards and Technology (NIST) Chemistry WebBook provide boiling point data for a wide range of compounds.
12.2 Software and Apps
Software and apps like ChemDraw and PubChem offer tools for predicting boiling points based on molecular structure.
13. Advanced Topics in Boiling Point
Advanced topics include azeotropes and vacuum distillation, which are crucial for separating mixtures with similar boiling points.
13.1 Azeotropes
Azeotropes are mixtures of liquids that boil at a constant temperature and composition, making them difficult to separate by обычная distillation.
13.2 Vacuum Distillation
Vacuum distillation lowers the boiling points of liquids by reducing the pressure, allowing for the separation of heat-sensitive compounds.
14. Common Mistakes to Avoid When Comparing Boiling Points
Avoiding common mistakes ensures accurate predictions and comparisons of boiling points.
14.1 Overlooking Intermolecular Forces
Failing to consider all types of intermolecular forces can lead to inaccurate predictions.
14.2 Ignoring Molecular Shape
Ignoring molecular shape and symmetry can result in incorrect assumptions about intermolecular interactions.
15. Real-World Applications of Boiling Point Knowledge
Boiling point knowledge is essential in various real-world applications, including distillation processes and solvent selection.
15.1 Distillation Processes
Distillation relies on differences in boiling points to separate liquids, used in industries ranging from petroleum refining to alcohol production.
15.2 Solvent Selection
Selecting the right solvent for a reaction or extraction process depends on its boiling point, ensuring efficient separation and purification.
16. Expert Insights on Comparing Boiling Points
Insights from experienced chemists provide valuable perspectives on comparing boiling points.
16.1 Tips from Experienced Chemists
Experienced chemists emphasize the importance of understanding intermolecular forces and considering molecular shape for accurate predictions.
16.2 Case Studies
Case studies illustrate the application of boiling point knowledge in solving real-world problems in chemistry and related fields.
17. Future Trends in Boiling Point Research
Future trends include the study of nanomaterials and the development of green chemistry techniques.
17.1 Nanomaterials
Research on nanomaterials explores how their unique properties affect boiling points and phase transitions.
17.2 Green Chemistry
Green chemistry focuses on developing sustainable and environmentally friendly methods for chemical processes, including distillation and separation.
18. Practical Tips for Students and Professionals
Practical tips for students and professionals include effective study techniques and opportunities for professional development.
18.1 Study Techniques
Effective study techniques include creating flashcards, practicing problem-solving, and reviewing key concepts regularly.
18.2 Professional Development
Professional development opportunities include attending conferences, taking advanced courses, and staying updated on the latest research.
19. Frequently Asked Questions (FAQs) About Boiling Points
Q1: What are the main factors affecting boiling point?
The main factors include intermolecular forces (ionic, hydrogen bonding, dipole-dipole, Van der Waals), molecular weight, and molecular symmetry. Stronger forces and higher molecular weights increase boiling point, while symmetry can decrease it.
Q2: How does hydrogen bonding affect boiling point?
Hydrogen bonding significantly increases boiling point because it is a strong intermolecular force that requires more energy to overcome. Compounds with hydrogen bonds generally have much higher boiling points than similar compounds without them.
Q3: Why do larger molecules have higher boiling points?
Larger molecules have higher boiling points because they have more electrons and greater surface area, leading to stronger Van der Waals dispersion forces.
Q4: What is the difference between boiling point and melting point?
Boiling point is the temperature at which a liquid turns into a gas, while melting point is the temperature at which a solid turns into a liquid. They reflect different phase transitions and different types of intermolecular forces.
Q5: How can I predict the boiling point of a compound?
Identify the intermolecular forces present, consider the molecular weight, and evaluate the molecular symmetry. Use this information to estimate the boiling point relative to similar compounds with known boiling points.
Q6: What are azeotropes?
Azeotropes are mixtures of liquids that boil at a constant temperature and composition, making them difficult to separate by ordinary distillation.
Q7: What is vacuum distillation and why is it used?
Vacuum distillation lowers the boiling points of liquids by reducing the pressure, allowing for the separation of heat-sensitive compounds that would decompose at their normal boiling points.
Q8: How does branching affect boiling point?
Branching generally decreases boiling point because it reduces the surface area available for intermolecular interactions, particularly Van der Waals forces.
Q9: Where can I find reliable boiling point data for different compounds?
You can find reliable boiling point data in online databases such as the NIST Chemistry WebBook and chemical handbooks like the CRC Handbook of Chemistry and Physics.
Q10: Why is boiling point important in chemistry and chemical engineering?
Boiling point is crucial for distillation, separation, and purification processes in chemistry and chemical engineering, as it allows for the isolation of specific compounds from mixtures.
20. Conclusion: Mastering the Art of Boiling Point Comparison with COMPARE.EDU.VN
Understanding and comparing boiling points requires a solid grasp of intermolecular forces, molecular weight, and symmetry. By following the guidelines and utilizing the resources provided by COMPARE.EDU.VN, students, professionals, and anyone curious can accurately predict and compare boiling points, unlocking a deeper understanding of chemistry and its applications. Remember to consider all relevant factors and utilize available tools to refine your predictions and solve complex problems. Whether you’re working in a lab, studying for an exam, or simply curious, COMPARE.EDU.VN is your trusted resource for mastering the art of boiling point comparison. Visit compare.edu.vn today at 333 Comparison Plaza, Choice City, CA 90210, United States, or contact us on Whatsapp at +1 (626) 555-9090. We provide detailed and objective comparisons of various scientific concepts, including boiling points, to help you make informed decisions.