Comparing IC50 values is crucial in pharmacology, drug discovery, and related fields to assess the potency of different compounds. At COMPARE.EDU.VN, we help you understand and accurately compare these values to make informed decisions. This guide will explore the methodologies, statistical analysis, and common pitfalls in IC50 comparisons, empowering you to interpret data effectively and draw meaningful conclusions about drug efficacy and resistance.
1. Understanding IC50 Values
1.1. What is IC50?
The IC50 (half-maximal inhibitory concentration) represents the concentration of a substance (e.g., a drug) required to inhibit a specific biological or biochemical function by 50%. It’s a quantitative measure of a drug’s potency, indicating how much of the drug is needed to achieve a particular effect.
1.2. Importance of IC50 in Drug Discovery
IC50 values are vital in the drug discovery process for several reasons:
- Potency Comparison: Enables comparison of the potency of different drugs targeting the same biological pathway or target.
- Dose Determination: Helps in determining appropriate dosages for in vitro and in vivo studies.
- Resistance Monitoring: Used to monitor the development of drug resistance in pathogens or cancer cells.
- Structure-Activity Relationship (SAR): Aids in understanding the relationship between a drug’s structure and its activity.
Alt Text: A sigmoidal dose-response curve illustrating the relationship between drug concentration and its inhibitory effect, highlighting the IC50 point at 50% inhibition.
2. Methodologies for Determining IC50
2.1. In Vitro Assays
IC50 values are typically determined through in vitro assays, where the drug’s effect is measured in a controlled laboratory setting. Common assays include:
- Enzyme Inhibition Assays: Measures the drug’s ability to inhibit a specific enzyme’s activity.
- Cell-Based Assays: Assesses the drug’s effect on cell proliferation, viability, or signaling pathways.
- Receptor Binding Assays: Determines the drug’s affinity for a specific receptor.
2.2. Curve Fitting Methods
To calculate IC50 values, raw data from these assays are plotted against drug concentrations, and a dose-response curve is generated. Curve fitting software, such as GraphPad Prism or similar tools, is then used to fit the data to a sigmoidal curve. The IC50 value is derived from this curve.
2.3. Point-to-Point Analysis
Alternatively, a point-to-point analysis can be performed, especially when curve-fitting software is unavailable. This method involves plotting the raw data and estimating the IC50 value directly from the graph. While less precise, it can provide a reasonable estimate.
3. Factors Affecting IC50 Values
Several factors can influence IC50 values, impacting the accuracy and comparability of results.
3.1. Assay Conditions
Assay conditions such as temperature, pH, buffer composition, and incubation time can significantly affect IC50 values. It’s crucial to maintain consistent conditions across experiments to ensure reliable results.
3.2. Cell Type and Passage Number
When using cell-based assays, the cell type and passage number can influence IC50 values. Different cell lines may exhibit varying sensitivities to the same drug due to differences in target expression, metabolic activity, or signaling pathways.
3.3. Serum Concentration
Serum concentration in the culture medium can also affect IC50 values. Serum proteins can bind to the drug, reducing the amount of free drug available to interact with the target.
3.4. Solvent Effects
The solvent used to dissolve the drug can influence IC50 values, particularly if the solvent affects the drug’s solubility or stability. It’s important to use a solvent that does not interfere with the assay.
4. Statistical Analysis of IC50 Data
4.1. Importance of Statistical Analysis
Statistical analysis is essential for determining the significance of differences in IC50 values and for identifying outliers or trends in the data.
4.2. Common Statistical Methods
- T-tests: Used to compare the means of two groups.
- ANOVA (Analysis of Variance): Used to compare the means of more than two groups.
- Regression Analysis: Used to assess the relationship between drug concentration and inhibitory effect.
4.3. Identifying Outliers
Outliers can significantly skew the results and should be identified and addressed appropriately. Common methods for identifying outliers include:
- Box Plots: Visual representation of the data distribution, highlighting potential outliers.
- Grubb’s Test: Statistical test for detecting a single outlier in a dataset.
- Chauvenet’s Criterion: Another statistical method for identifying outliers based on probability.
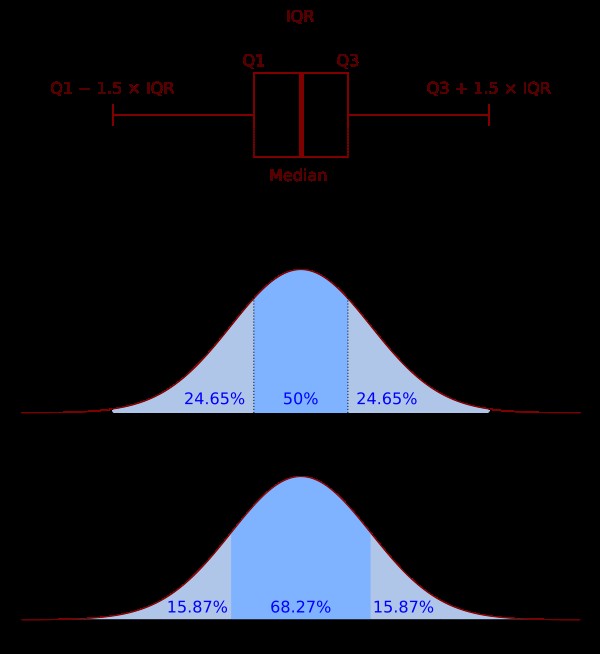 Box Plot
Box Plot
Alt Text: A box plot illustrating the distribution of IC50 values, with whiskers indicating the range and outliers marked beyond the whiskers.
5. Interpreting IC50 Values
5.1. Understanding the Range of IC50 Values
IC50 values can vary widely depending on the drug, target, and assay conditions. It’s important to understand the typical range of IC50 values for a given drug and target to interpret the results accurately.
5.2. Comparing IC50 Values Across Different Studies
When comparing IC50 values across different studies, it’s crucial to consider the differences in assay conditions, cell types, and statistical methods. Direct comparisons may not be valid if these factors are not standardized.
5.3. Clinical Relevance of IC50 Values
IC50 values obtained in vitro may not always translate directly to clinical efficacy. Factors such as drug metabolism, distribution, and clearance can influence the drug’s concentration at the target site in vivo. Therefore, it’s important to consider these factors when interpreting the clinical relevance of IC50 values.
6. Common Pitfalls in IC50 Comparisons
6.1. Ignoring Assay Variability
Failing to account for assay variability can lead to inaccurate comparisons of IC50 values. It’s important to perform multiple replicates and use appropriate statistical methods to assess the variability of the data.
6.2. Over-Interpreting Small Differences
Small differences in IC50 values may not be statistically significant or clinically relevant. It’s important to consider the magnitude of the difference and its potential impact on drug efficacy.
6.3. Neglecting the Mechanism of Action
IC50 values only provide information about the potency of a drug, not its mechanism of action. It’s important to consider the drug’s mechanism of action when interpreting IC50 values and comparing different drugs.
6.4. Improper Curve Fitting
Incorrect curve fitting can lead to inaccurate IC50 values. It’s important to use appropriate curve fitting software and to validate the curve fit by visually inspecting the data.
7. Optimizing IC50 Determination
7.1. Standardizing Assay Conditions
Standardizing assay conditions is crucial for obtaining reliable and reproducible IC50 values. This includes controlling temperature, pH, buffer composition, and incubation time.
7.2. Using Appropriate Controls
Appropriate controls should be included in each experiment to account for background noise and non-specific effects. These controls may include vehicle-treated samples, positive controls, and negative controls.
7.3. Performing Replicates
Performing multiple replicates is essential for assessing the variability of the data and for obtaining statistically significant results.
7.4. Validating the Assay
The assay should be validated to ensure that it is measuring the intended biological or biochemical function accurately and reproducibly. This may involve testing known inhibitors or activators of the target.
8. IC50 in Drug Resistance Studies
8.1. Monitoring Drug Resistance
IC50 values are commonly used to monitor the development of drug resistance in pathogens or cancer cells. An increase in IC50 values over time may indicate the emergence of resistance.
8.2. Mechanisms of Resistance
Drug resistance can arise through various mechanisms, including:
- Target Mutation: Mutations in the drug target that reduce its affinity for the drug.
- Increased Drug Efflux: Increased expression of efflux pumps that remove the drug from the cell.
- Decreased Drug Uptake: Reduced expression of drug transporters that facilitate drug entry into the cell.
- Bypass Pathways: Activation of alternative signaling pathways that bypass the drug target.
8.3. Determining Resistance Cut-Off Values
Determining resistance cut-off values is important for identifying resistant isolates or cell lines. Commonly used criteria include:
- Values Greater Than 3 Standard Deviations (SD) from the Mean: Indicates a significant deviation from the normal range.
- Values 10-Fold or Greater Than the Mean: Suggests a substantial increase in resistance.
9. Examples and Case Studies
9.1. Case Study 1: Comparing Inhibitors of Enzyme X
Consider a scenario where you are comparing two inhibitors (Inhibitor A and Inhibitor B) of Enzyme X. The IC50 values are determined using an enzyme inhibition assay.
| Inhibitor | IC50 Value (nM) |
|---|---|
| Inhibitor A | 5 |
| Inhibitor B | 25 |
In this case, Inhibitor A has a lower IC50 value (5 nM) compared to Inhibitor B (25 nM), indicating that Inhibitor A is more potent in inhibiting Enzyme X.
9.2. Case Study 2: Monitoring Drug Resistance in Cancer Cells
A cancer research lab is monitoring the resistance of cancer cells to a chemotherapeutic drug over time. The IC50 values are measured at different time points.
| Time Point | IC50 Value (μM) |
|---|---|
| Initial | 0.5 |
| 6 Months | 2.0 |
| 12 Months | 10.0 |
The increasing IC50 values over time indicate that the cancer cells are developing resistance to the chemotherapeutic drug. Further investigation is needed to understand the mechanisms of resistance.
10. Tools and Resources for IC50 Analysis
10.1. Curve Fitting Software
- GraphPad Prism: Widely used software for curve fitting and statistical analysis.
- SigmaPlot: Another popular software for scientific graphing and data analysis.
- Origin: Software for data analysis and technical graphing.
10.2. Online IC50 Calculators
- AAT Bioquest IC50 Calculator: Online tool for calculating IC50 values.
- MyAssays: Web-based platform for data analysis and curve fitting.
10.3. Statistical Software
- R: A programming language and free software environment for statistical computing and graphics.
- SPSS: Statistical Package for the Social Sciences, a software suite used for statistical analysis.
Alt Text: The GraphPad Prism logo, representing a widely used software for curve fitting and statistical analysis of IC50 values.
11. Advanced Techniques in IC50 Determination
11.1. High-Throughput Screening (HTS)
HTS is a method for rapidly screening a large number of compounds for their ability to inhibit a specific target. IC50 values are typically determined for the most promising compounds identified in the screen.
11.2. Surface Plasmon Resonance (SPR)
SPR is a technique for measuring the binding affinity of a drug to its target in real-time. SPR can be used to determine IC50 values by measuring the drug’s ability to inhibit the binding of a ligand to the target.
11.3. Isothermal Titration Calorimetry (ITC)
ITC is a technique for measuring the heat released or absorbed during a binding event. ITC can be used to determine IC50 values by measuring the drug’s ability to inhibit the binding of a ligand to the target.
12. Future Trends in IC50 Analysis
12.1. Machine Learning
Machine learning algorithms are being used to predict IC50 values based on chemical structure and other properties of the drug. This can help accelerate the drug discovery process by identifying promising compounds for further testing.
12.2. Artificial Intelligence (AI)
AI is being used to automate the analysis of IC50 data and to identify patterns or trends that may not be apparent to human researchers. This can help improve the accuracy and efficiency of IC50 analysis.
12.3. Integration with Big Data
IC50 data is being integrated with other types of data, such as genomic, proteomic, and clinical data, to gain a more comprehensive understanding of drug efficacy and resistance. This can help personalize drug therapy and improve patient outcomes.
13. Conclusion: Leveraging COMPARE.EDU.VN for Informed Decisions
Comparing IC50 values accurately is crucial for making informed decisions in drug discovery, pharmacology, and related fields. At COMPARE.EDU.VN, we understand the challenges involved and provide the resources and expertise you need to interpret IC50 data effectively. By understanding the methodologies, statistical analysis, and common pitfalls in IC50 comparisons, you can draw meaningful conclusions about drug efficacy and resistance.
Navigating the Complexities of IC50 Comparison
IC50 values are essential for assessing drug potency and resistance, but their interpretation requires careful consideration of various factors, including assay conditions, statistical methods, and potential sources of variability. Trust COMPARE.EDU.VN to guide you through these complexities.
Empowering Your Research and Decisions
Whether you’re a researcher, clinician, or student, COMPARE.EDU.VN offers a comprehensive suite of tools and information to help you compare IC50 values accurately and make informed decisions. Our resources include detailed guides, case studies, and access to expert analysis.
Your Go-To Resource for Comparative Analysis
Turn to COMPARE.EDU.VN for all your comparative analysis needs. We are committed to providing objective, reliable, and up-to-date information to help you navigate the complex world of scientific data.
14. Frequently Asked Questions (FAQ) on IC50 Data Analysis
Q: What IC50 values can I expect to get?
A: Susceptibility to NI drugs is not absolute. There are typical ranges of IC50 values that differ between influenza subtypes and between oseltamivir and zanamivir for each subtype. Therefore this subtype and drug-specific data should not be compared. Influenza B viruses tend to have IC50 values 10-100 fold higher than influenza A viruses.
Q: How do I determine IC50?
A: Raw data (relative fluorescence or luminescence units) are plotted against the drug concentration. IC50 values can be determined in two ways; using curve-fitting software, or by a point-to-point analysis. Several curve-fitting software is commercially available: Graphpad Prism, Grafit.
Q: A 2-3-fold difference is seen when the same virus is re-tested. Is this normal?
A: A 2-3-fold difference in IC50 values is common, in all assay methodologies.
Q: What IC50 value obtained in a neuraminidase inhibition assay can be used as a cut-off value for clinically relevant resistance?
A: There is no firm definition of a resistant IC50. Commonly used criteria to identify isolates that outside the normal range are either: 1. A value greater than 3SD from the mean (or median) value for the given subtype and drug. 2. A value 10-fold or greater than the mean (or median) value for the given subtype and drug.
Q: What should I do with intermediate IC50 results?
A: Intermediate results are those which fall over the minor cut-off (whether using box and whisker or SMAD to monitor IC50s) and under the major outlier cut-off and therefore are not strictly termed as resistant. The IC50 test for such isolates should be repeated to confirm the result, and further characterized where possible, for example, sequencing of the NA gene.
Q: Could drug susceptibility assessment be based solely on the result of the neuraminidase inhibition assay provided that the IC50 value is very high or rather low?
A: No, such a practice is not recommended. Genotypic analysis such as Sanger sequencing, or pyrosequencing may be needed, including on matching clinical specimens.
Q: Some ‘drug-resistant’ reference influenza A viruses (e.g., E119V in H3N2) have IC50 values that are not very different from the IC50 values of some ‘drug-sensitive’ influenza B viruses. Why is this?
A: A comparison needs to be done for each specific subtype and type. The chemiluminescent assay is less discriminative compared to the fluorescent assay. In addition, influenza B viruses have been shown to be less susceptible to oseltamivir in a clinical trial.
Q: Some influenza viruses show an elevated IC50 value but they do not have any known or unique changes in the NA. What is the reason for the elevated IC50 value?
A: There could be several reasons for this: 1. Repeat the test. 2. Virus preparation may contain a mix of influenza A and B viruses and influenza B viruses typically have higher IC50 values. It is prudent to perform real-time RT-PCR testing to subtype the virus isolate. 3. There could be a mix of virus variants and cloning may help to separate a resistant variant from a sensitive one.
Q: How do I analyze my IC50 values over time?
A: Once you have tested a number of isolates of a given subtype against a drug, you can determine the ‘normal range’. This can be done in two ways: Box Plot Analysis and robust statistics using the standard deviation of the median absolute deviation of the median (SMAD).
Q: How should I monitor trends?
A: Trends from one influenza season to the next can be monitored using the box and whisker or SMAD method to determine whether the mean/median values and the minor and major cut-off criteria are changing or remaining approximately constant.
15. Call to Action
Ready to make informed decisions about drug potency and resistance? Visit COMPARE.EDU.VN today to explore our comprehensive resources on IC50 comparison. Our detailed guides, expert analysis, and user-friendly tools will empower you to interpret data effectively and drive meaningful results.
Don’t navigate the complexities of IC50 analysis alone. Trust COMPARE.EDU.VN to guide you.
Visit COMPARE.EDU.VN now and unlock the power of informed decision-making.
Address: 333 Comparison Plaza, Choice City, CA 90210, United States
Whatsapp: +1 (626) 555-9090
Website: compare.edu.vn