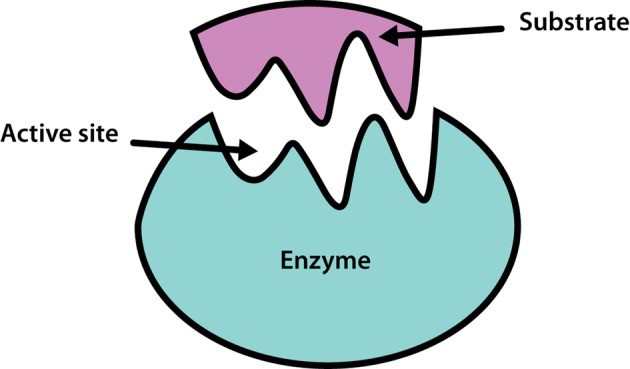
Enzymes are often compared to a lock and key, a concept crucial for understanding their function and specificity. COMPARE.EDU.VN delves into this analogy, explaining how it illuminates the enzyme-substrate interaction and highlighting the role of enzymes in biological reactions. Exploring this enzymatic action is key to understanding biochemistry and its wide-ranging applications.
1. Understanding the Lock and Key Analogy for Enzymes
Enzymes, the biological catalysts, accelerate chemical reactions within living organisms. Their function is frequently described using the lock and key analogy. This model helps explain how enzymes selectively bind to specific substrates. This discussion will illuminate the simplicity and explanatory power of this concept.
1.1. The Original Lock and Key Hypothesis
Emil Fischer introduced the lock and key hypothesis in 1894, suggesting that an enzyme’s active site has a specific shape that perfectly matches the shape of its substrate, much like a lock only accepts a specific key. This initial hypothesis laid the groundwork for understanding enzyme specificity.
1.2. How the Lock and Key Model Works
The lock and key model visualizes the enzyme as the lock and the substrate as the key. Only the correct substrate (key) can fit into the enzyme’s active site (lock). This precise fit ensures that the enzyme catalyzes a specific reaction involving that substrate.
1.3. Strengths of the Analogy
- Simplicity: The lock and key analogy is easy to understand, making it a useful tool for introducing enzyme function.
- Specificity: It effectively illustrates why enzymes are highly specific, catalyzing reactions with only certain substrates.
- Foundation: It provides a foundational understanding of enzyme-substrate interactions.
1.4. Limitations of the Analogy
The lock and key model is a simplification of the complex reality of enzyme function. It fails to account for the dynamic nature of enzymes and the changes they undergo upon substrate binding. A more accurate model, known as the induced fit model, addresses these limitations.
2. The Induced Fit Model: A Refined Understanding
The induced fit model builds upon the lock and key analogy, offering a more comprehensive explanation of enzyme-substrate interactions. This model recognizes that enzymes are flexible molecules that can change shape upon substrate binding. This allows for greater specificity and catalytic efficiency.
2.1. Introduction to the Induced Fit Model
Daniel Koshland proposed the induced fit model in 1958. Unlike the rigid lock and key model, the induced fit model suggests that the enzyme’s active site is not perfectly complementary to the substrate initially. Instead, both the enzyme and substrate undergo conformational changes to achieve optimal binding.
2.2. How the Induced Fit Model Works
When a substrate approaches an enzyme, it induces a change in the enzyme’s active site. This conformational change allows the enzyme to bind more tightly to the substrate, forming an enzyme-substrate complex. The enzyme essentially molds itself around the substrate to achieve the best possible fit.
2.3. Advantages of the Induced Fit Model
- Flexibility: The induced fit model accounts for the flexibility of enzymes and their ability to adapt to different substrates.
- Specificity: It explains how enzymes can catalyze reactions with a range of similar substrates, as the enzyme can adjust its active site to accommodate slight variations.
- Catalysis: The conformational changes induced by substrate binding can promote the catalytic process by straining bonds in the substrate or bringing catalytic groups into proximity.
2.4. Comparison with the Lock and Key Model
While the lock and key model provides a basic understanding of enzyme specificity, the induced fit model offers a more nuanced and accurate depiction of enzyme function. The induced fit model recognizes the dynamic nature of enzymes and their ability to adapt to their substrates, leading to more effective catalysis.
3. The Active Site: Where the Magic Happens
The active site is the specific region on an enzyme where the substrate binds and the chemical reaction takes place. It is a three-dimensional pocket or groove formed by amino acid residues that create a unique microenvironment for catalysis. Understanding the active site is key to understanding how enzymes work.
3.1. Location and Structure of the Active Site
The active site is usually a small portion of the total enzyme structure, often located in a cleft or crevice on the enzyme surface. It is formed by amino acid residues that may be far apart in the primary sequence but are brought together by the enzyme’s three-dimensional folding.
3.2. Amino Acid Residues Involved in Catalysis
The amino acid residues in the active site are crucial for substrate binding and catalysis. Some residues are involved in binding the substrate through hydrogen bonds, hydrophobic interactions, or ionic bonds. Other residues act as catalysts, either by donating or accepting protons, forming temporary covalent bonds with the substrate, or stabilizing the transition state.
3.3. Substrate Binding
The substrate binds to the active site with high affinity and specificity. This binding is driven by various forces, including:
- Hydrogen bonds: Formed between polar amino acid residues and polar groups on the substrate.
- Hydrophobic interactions: Occur between nonpolar amino acid residues and hydrophobic regions of the substrate.
- Ionic bonds: Formed between charged amino acid residues and oppositely charged groups on the substrate.
- Van der Waals forces: Weak attractions between atoms in close proximity.
3.4. Catalytic Mechanisms
Once the substrate is bound to the active site, the enzyme employs various catalytic mechanisms to accelerate the reaction. These mechanisms include:
- Acid-base catalysis: Amino acid residues act as acids or bases to donate or accept protons, stabilizing transition states.
- Covalent catalysis: The enzyme forms a temporary covalent bond with the substrate, creating a reactive intermediate.
- Metal ion catalysis: Metal ions in the active site participate in catalysis by stabilizing charged intermediates or facilitating redox reactions.
- Proximity and orientation effects: The enzyme brings the substrates into close proximity and optimal orientation for the reaction to occur.
- Transition state stabilization: The enzyme preferentially binds to and stabilizes the transition state, lowering the activation energy of the reaction.
4. Factors Affecting Enzyme Activity
Enzyme activity is influenced by a variety of factors, including temperature, pH, substrate concentration, and the presence of inhibitors or activators. Understanding these factors is crucial for optimizing enzyme function in both biological systems and industrial applications.
4.1. Temperature
Temperature affects enzyme activity in several ways. As temperature increases, the rate of enzyme-catalyzed reactions generally increases due to increased molecular motion and collision frequency. However, beyond a certain temperature, the enzyme begins to denature, losing its three-dimensional structure and activity. Each enzyme has an optimal temperature at which its activity is maximal.
4.2. pH
pH affects the ionization state of amino acid residues in the active site and the substrate, which can influence substrate binding and catalysis. Each enzyme has an optimal pH at which its activity is maximal. Changes in pH can also lead to enzyme denaturation.
4.3. Substrate Concentration
As substrate concentration increases, the rate of enzyme-catalyzed reactions generally increases until a maximum velocity (Vmax) is reached. At Vmax, the enzyme is saturated with substrate, and further increases in substrate concentration have no effect on the reaction rate. The Michaelis-Menten constant (Km) is a measure of the substrate concentration required to achieve half of Vmax and is an indicator of the enzyme’s affinity for its substrate.
4.4. Enzyme Concentration
The rate of an enzyme-catalyzed reaction is directly proportional to the enzyme concentration, provided that substrate is present in excess. Increasing the enzyme concentration increases the number of active sites available to bind substrate, leading to a higher reaction rate.
4.5. Inhibitors
Inhibitors are substances that reduce enzyme activity. They can be classified as:
- Competitive inhibitors: Bind to the active site, preventing substrate binding.
- Noncompetitive inhibitors: Bind to a site on the enzyme distinct from the active site, altering the enzyme’s conformation and reducing its activity.
- Uncompetitive inhibitors: Bind to the enzyme-substrate complex, preventing the release of product.
4.6. Activators
Activators are substances that increase enzyme activity. They can bind to the enzyme and induce a conformational change that enhances substrate binding or catalysis.
5. Enzyme Classification
Enzymes are classified into six major classes based on the type of reaction they catalyze. Each class is further divided into subclasses and sub-subclasses, providing a hierarchical system for organizing and naming enzymes. Understanding enzyme classification helps to categorize and understand their diverse functions.
5.1. Oxidoreductases
Oxidoreductases catalyze oxidation-reduction reactions, transferring electrons from one molecule (the reductant) to another (the oxidant). These enzymes often involve cofactors such as NAD+ or FAD.
- Examples: Dehydrogenases, oxidases, reductases
5.2. Transferases
Transferases catalyze the transfer of a functional group (e.g., methyl, phosphate, amino) from one molecule to another.
- Examples: Kinases, transaminases
5.3. Hydrolases
Hydrolases catalyze the hydrolysis of chemical bonds, adding water to break the bond.
- Examples: Esterases, peptidases, glycosidases
5.4. Lyases
Lyases catalyze the breaking of chemical bonds by means other than hydrolysis or oxidation, often forming a double bond or a new ring structure.
- Examples: Decarboxylases, dehydratases
5.5. Isomerases
Isomerases catalyze the conversion of one isomer to another, rearranging the atoms within a molecule.
- Examples: Epimerases, racemases
5.6. Ligases
Ligases catalyze the joining of two molecules, coupled with the hydrolysis of ATP or another nucleoside triphosphate.
- Examples: DNA ligase, aminoacyl-tRNA synthetase
6. Applications of Enzymes
Enzymes have a wide range of applications in various industries, including food processing, pharmaceuticals, diagnostics, and environmental remediation. Their specificity and efficiency make them valuable tools for a variety of processes.
6.1. Food Industry
Enzymes are used in the food industry for various purposes, including:
- Starch hydrolysis: Amylases are used to break down starch into sugars in the production of syrups and sweeteners.
- Protein hydrolysis: Proteases are used to tenderize meat and improve the texture of baked goods.
- Lactose hydrolysis: Lactase is used to produce lactose-free dairy products.
- Cheese production: Rennin is used to coagulate milk in cheese making.
- Fruit juice clarification: Pectinases are used to clarify fruit juices by breaking down pectin.
6.2. Pharmaceutical Industry
Enzymes are used in the pharmaceutical industry for:
- Drug synthesis: Enzymes are used as biocatalysts in the synthesis of various drugs, including antibiotics and steroids.
- Drug targeting: Enzymes are used to target drugs to specific tissues or cells.
- Enzyme inhibitors as drugs: Certain drugs act as enzyme inhibitors, targeting specific enzymes involved in disease pathways.
6.3. Diagnostics
Enzymes are widely used in diagnostic assays for:
- Measuring glucose levels: Glucose oxidase is used in blood glucose meters for monitoring diabetes.
- Detecting disease markers: Enzymes are used in enzyme-linked immunosorbent assays (ELISAs) to detect antibodies or antigens in blood or other body fluids.
- Clinical chemistry: Enzymes are used to measure the levels of various metabolites and enzymes in blood and urine for diagnosing and monitoring diseases.
6.4. Environmental Remediation
Enzymes are used in environmental remediation for:
- Degrading pollutants: Enzymes are used to break down pollutants in soil and water, such as pesticides, herbicides, and industrial chemicals.
- Bioremediation: Enzymes are used to enhance the bioremediation of contaminated sites by accelerating the degradation of pollutants by microorganisms.
6.5. Textile Industry
Enzymes have been used in the textile industry for a variety of purposes, including:
- Bio-polishing: Cellulases are used to remove fuzz and pills from cotton fabrics, resulting in a smoother and brighter fabric surface.
- Desizing: Amylases are used to remove starch-based sizing agents from fabrics, improving their softness and absorbency.
- Denim washing: Cellulases are used to create a worn or faded look in denim fabrics.
- Wool scouring: Proteases are used to remove impurities from wool fibers, improving their softness and luster.
These applications of enzymes highlight their importance in various industries and their potential for future innovations.
7. Enzyme Immobilization
Enzyme immobilization is the process of attaching enzymes to a solid support, allowing them to be reused and increasing their stability. This technique has significant advantages for industrial applications, making enzymatic processes more efficient and cost-effective.
7.1. Methods of Enzyme Immobilization
There are several methods for enzyme immobilization, including:
- Adsorption: Enzymes are adsorbed onto the surface of a solid support, such as activated carbon or alumina.
- Entrapment: Enzymes are entrapped within a matrix, such as a gel or a fiber.
- Covalent bonding: Enzymes are covalently bonded to a solid support, creating a strong and stable attachment.
- Cross-linking: Enzymes are cross-linked to each other, forming aggregates that are insoluble and easy to recover.
7.2. Advantages of Enzyme Immobilization
- Reusability: Immobilized enzymes can be reused multiple times, reducing the cost of enzyme-based processes.
- Increased stability: Immobilization can protect enzymes from denaturation, increasing their stability and activity over time.
- Easy separation: Immobilized enzymes can be easily separated from the reaction mixture, simplifying product recovery.
- Continuous operation: Immobilized enzymes can be used in continuous flow reactors, allowing for continuous production.
7.3. Applications of Immobilized Enzymes
Immobilized enzymes are used in a variety of industrial processes, including:
- High-fructose corn syrup production: Glucose isomerase is immobilized to produce high-fructose corn syrup from glucose.
- Lactose-free milk production: Lactase is immobilized to hydrolyze lactose in milk, producing lactose-free dairy products.
- Acrylamide production: Nitrile hydratase is immobilized to produce acrylamide from acrylonitrile.
- Semi-synthetic penicillin production: Penicillin acylase is immobilized to produce 6-aminopenicillanic acid (6-APA), a precursor for semi-synthetic penicillins.
8. Enzyme Engineering
Enzyme engineering is the process of modifying the structure and function of enzymes to improve their properties for industrial or therapeutic applications. This can be achieved through various techniques, including site-directed mutagenesis, directed evolution, and computational design.
8.1. Site-Directed Mutagenesis
Site-directed mutagenesis involves introducing specific mutations into the gene encoding an enzyme, altering the amino acid sequence and potentially modifying its properties. This technique is used to:
- Improve enzyme activity: By optimizing the active site for substrate binding and catalysis.
- Increase enzyme stability: By introducing mutations that protect the enzyme from denaturation.
- Alter substrate specificity: By modifying the active site to accommodate different substrates.
- Reduce enzyme inhibition: By removing or modifying binding sites for inhibitors.
8.2. Directed Evolution
Directed evolution involves creating a library of enzyme variants through random mutagenesis or recombination, followed by screening or selection for enzymes with improved properties. This technique mimics natural evolution in the laboratory and can be used to:
- Improve enzyme activity: By selecting for enzymes with higher catalytic rates.
- Increase enzyme stability: By selecting for enzymes that are more resistant to denaturation.
- Alter substrate specificity: By selecting for enzymes that can utilize different substrates.
- Enhance enzyme expression: By selecting for enzymes that are produced at higher levels.
8.3. Computational Enzyme Design
Computational enzyme design involves using computer modeling and simulation to design enzymes with desired properties. This technique can be used to:
- Design new enzymes: By creating enzymes that catalyze novel reactions.
- Improve existing enzymes: By optimizing the structure of existing enzymes for improved activity, stability, or substrate specificity.
- Predict enzyme properties: By using computer models to predict the properties of enzyme variants.
9. E-E-A-T and YMYL Considerations for Enzyme-Related Content
Creating content about enzymes requires careful consideration of Expertise, Experience, Authoritativeness, and Trustworthiness (E-E-A-T) as well as Your Money or Your Life (YMYL) principles, especially when discussing applications related to health or medicine.
9.1. Demonstrating Expertise
Content should be accurate, detailed, and reflect a deep understanding of enzymology. This means:
- Providing clear explanations of complex processes such as enzyme kinetics, mechanisms of action, and the effects of inhibitors.
- Citing reputable sources, including peer-reviewed scientific articles, textbooks, and authoritative websites.
- Ensuring that all claims are supported by scientific evidence.
9.2. Showcasing Experience
Where possible, incorporate real-world examples and case studies to illustrate the applications of enzymes. This could include:
- Discussing specific industrial processes that utilize enzymes, such as the production of high-fructose corn syrup or lactose-free milk.
- Highlighting the use of enzymes in diagnostic assays and medical treatments.
- Sharing experiences from researchers or professionals working with enzymes.
9.3. Establishing Authoritativeness
Build authority by:
- Creating content that is recognized and referenced by others in the field.
- Participating in relevant discussions and forums, sharing your expertise and insights.
- Ensuring that the COMPARE.EDU.VN website is seen as a reliable source of information on enzyme-related topics.
9.4. Building Trustworthiness
Trustworthiness is paramount, especially when discussing health-related applications. Here’s how to build it:
- Provide clear disclaimers stating that the information is for educational purposes only and should not be considered medical advice.
- Ensure that all content is unbiased and objective, presenting both the benefits and limitations of enzyme-based approaches.
- Regularly update content to reflect the latest scientific findings and best practices.
9.5. YMYL Considerations
Content related to enzyme applications in health and medicine falls under YMYL because it can directly impact a person’s well-being. Therefore:
- Exercise extreme caution when discussing the therapeutic uses of enzymes, ensuring that all claims are supported by rigorous scientific evidence and regulatory approvals.
- Avoid promoting unproven or experimental treatments.
- Clearly differentiate between established medical practices and emerging research.
- Consult with medical professionals to review content and ensure accuracy.
By adhering to these E-E-A-T and YMYL principles, you can create enzyme-related content that is not only informative but also trustworthy and beneficial to your audience.
10. Frequently Asked Questions (FAQ) about Enzymes
Q1: What are enzymes and what do they do?
A1: Enzymes are biological catalysts, primarily proteins, that speed up chemical reactions in living organisms. They do this by lowering the activation energy required for the reaction to occur.
Q2: How do enzymes work?
A2: Enzymes work by binding to specific molecules called substrates at their active site. This binding forms an enzyme-substrate complex, which facilitates the chemical reaction. The enzyme then releases the products of the reaction, remaining unchanged and ready to catalyze another reaction.
Q3: What is the lock and key analogy?
A3: The lock and key analogy is a model that explains enzyme specificity. It suggests that an enzyme’s active site has a specific shape that perfectly matches the shape of its substrate, like a lock only accepts a specific key.
Q4: What is the induced fit model?
A4: The induced fit model is a more refined explanation of enzyme-substrate interactions. It suggests that the enzyme’s active site is not perfectly complementary to the substrate initially. Instead, both the enzyme and substrate undergo conformational changes to achieve optimal binding.
Q5: What factors affect enzyme activity?
A5: Enzyme activity is influenced by several factors, including temperature, pH, substrate concentration, enzyme concentration, and the presence of inhibitors or activators.
Q6: What are enzyme inhibitors?
A6: Enzyme inhibitors are substances that reduce enzyme activity. They can be competitive (binding to the active site) or noncompetitive (binding to a different site on the enzyme).
Q7: What are the main classes of enzymes?
A7: The main classes of enzymes are oxidoreductases, transferases, hydrolases, lyases, isomerases, and ligases.
Q8: What are some applications of enzymes?
A8: Enzymes have a wide range of applications in various industries, including food processing, pharmaceuticals, diagnostics, and environmental remediation.
Q9: What is enzyme immobilization?
A9: Enzyme immobilization is the process of attaching enzymes to a solid support, allowing them to be reused and increasing their stability.
Q10: What is enzyme engineering?
A10: Enzyme engineering is the process of modifying the structure and function of enzymes to improve their properties for industrial or therapeutic applications.
Enzymes, with their lock and key specificity or dynamic induced fit, are essential for life and technology. Visit COMPARE.EDU.VN at 333 Comparison Plaza, Choice City, CA 90210, United States, or contact us via Whatsapp at +1 (626) 555-9090 for more in-depth comparisons and resources.
Are you struggling to compare different enzymes or enzymatic processes? Do you need help understanding which enzyme is best for a specific application? Visit compare.edu.vn today to find comprehensive comparisons, expert reviews, and detailed information to help you make informed decisions. Our resources can help you choose the right enzyme for your needs. Address: 333 Comparison Plaza, Choice City, CA 90210, United States. Whatsapp: +1 (626) 555-9090.