Comparing the acidity of organic compounds can seem daunting, but it’s a fundamental skill in organic chemistry. At COMPARE.EDU.VN, we provide a comprehensive breakdown of the key factors that influence acidity, allowing you to confidently predict and compare the acidic strength of different molecules. Understanding these principles will empower you to analyze molecular structures and determine relative acidity, improving your problem-solving skills in chemistry. We will delve into the nuances of acid strength, conjugate base stability, and structural influences.
1. Understanding Acidity: The Basics
Acidity in organic chemistry refers to the ability of a compound to donate a proton (H+). The strength of an acid is quantified by its pKa value – a lower pKa indicates a stronger acid. In simpler terms, a stronger acid readily donates its proton, while a weaker acid holds onto it more tightly. Therefore, when comparing organic compounds, it’s essential to understand how molecular structure and properties affect their ability to release protons.
1.1. Brønsted-Lowry Definition of Acidity
The Brønsted-Lowry definition is the foundation for understanding acidity. It defines acids as proton donors and bases as proton acceptors. When an acid donates a proton, it forms its conjugate base. The stability of this conjugate base is crucial in determining the acidity of the original acid. A more stable conjugate base indicates a stronger acid, as it readily gives up its proton to form a stable anion. This concept is vital when comparing the acidity of different organic compounds.
1.2. pKa and Acid Strength
The pKa value is a quantitative measure of acid strength. It is defined as the negative logarithm (base 10) of the acid dissociation constant (Ka).
- Strong Acids: Have low and even negative pKa values (e.g., hydrochloric acid, pKa ≈ -7).
- Weak Acids: Have higher pKa values (e.g., acetic acid, pKa ≈ 4.76).
- Very Weak Acids: Have very high pKa values (e.g., ethanol, pKa ≈ 16).
The relationship between pKa and acid strength is inverse: the lower the pKa, the stronger the acid. This means that a compound with a pKa of 2 is a much stronger acid than a compound with a pKa of 7.
1.3. Conjugate Base Stability
As mentioned, the stability of the conjugate base is directly related to the acidity of the corresponding acid. Factors that stabilize the conjugate base will increase the acidity of the acid. This is because a stable conjugate base means the acid more readily gives up its proton. Understanding the factors that contribute to conjugate base stability is essential for predicting the acidity of organic compounds.
2. Five Key Factors Affecting Acidity (CARDIO)
To effectively compare the acidity of organic compounds, we can use a mnemonic known as CARDIO. This mnemonic helps us remember the five key factors that influence acidity: Charge, Atom, Resonance, Dipole Induction, and Orbitals. Let’s explore each of these factors in detail.
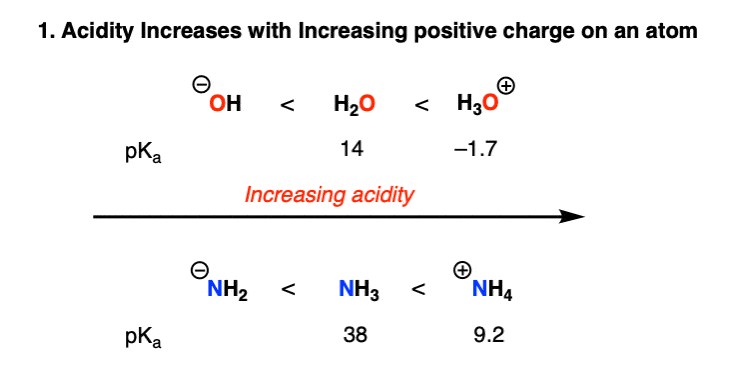
2.1. Charge: The Influence of Charge on Acidity
The charge on a molecule significantly affects its acidity. Positively charged molecules are more acidic because the loss of a proton (H+) reduces the positive charge, leading to a more stable state. Conversely, negatively charged molecules are less acidic because the loss of a proton would increase the negative charge, making the molecule less stable.
Examples:
- H3O+ (hydronium ion) is more acidic than H2O (water) because it carries a positive charge.
- H2O (water) is more acidic than HO- (hydroxide ion) because the hydroxide ion has a negative charge.
Table 1: Acidity Trends Based on Charge
| Compound | Charge | pKa | Relative Acidity |
|---|---|---|---|
| H3O+ | +1 | -1.7 | Highest |
| H2O | 0 | 15.7 | Intermediate |
| HO- | -1 | 36 | Lowest |
2.2. Atom: Electronegativity and Size
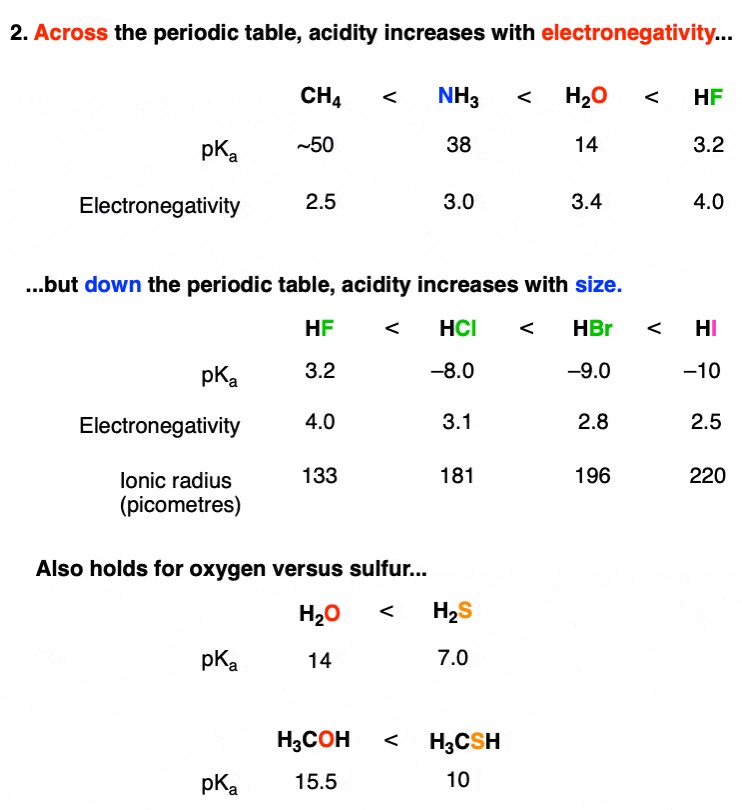
The type of atom bearing the acidic proton plays a significant role in determining acidity. Two main trends are observed: electronegativity and atomic size.
2.2.1. Electronegativity (Across a Row)
Across a row of the periodic table, acidity increases with increasing electronegativity. This is because more electronegative atoms are better at stabilizing the negative charge of the conjugate base. For example, consider the acidity of methane (CH4), ammonia (NH3), water (H2O), and hydrogen fluoride (HF).
- HF is more acidic than H2O
- H2O is more acidic than NH3
- NH3 is more acidic than CH4
This trend aligns with the increasing electronegativity of F > O > N > C. The more electronegative atom can better accommodate the negative charge resulting from the loss of a proton.
2.2.2. Atomic Size (Down a Group)
Down a group in the periodic table, acidity increases with increasing atomic size. This trend is due to the increased stability of the conjugate base as the negative charge is distributed over a larger volume. For instance, consider the hydrogen halides: HF, HCl, HBr, and HI.
- HI is a stronger acid than HBr
- HBr is a stronger acid than HCl
- HCl is a stronger acid than HF
Although fluorine is the most electronegative element, the fluoride ion (F-) is small, concentrating the negative charge. Larger halide ions like iodide (I-) have their charge dispersed over a larger volume, leading to greater stability and, consequently, higher acidity of HI.
Table 2: Acidity Trends Based on Atom Type
| Compound | Acidic Atom | Electronegativity | Atomic Size | pKa | Relative Acidity |
|---|---|---|---|---|---|
| CH4 | C | 2.55 | Small | ~50 | Very Low |
| NH3 | N | 3.04 | Small | ~36 | Low |
| H2O | O | 3.44 | Small | 15.7 | Moderate |
| HF | F | 3.98 | Small | 3.2 | High |
| HI | I | 2.66 | Large | -9.5 | Very High |
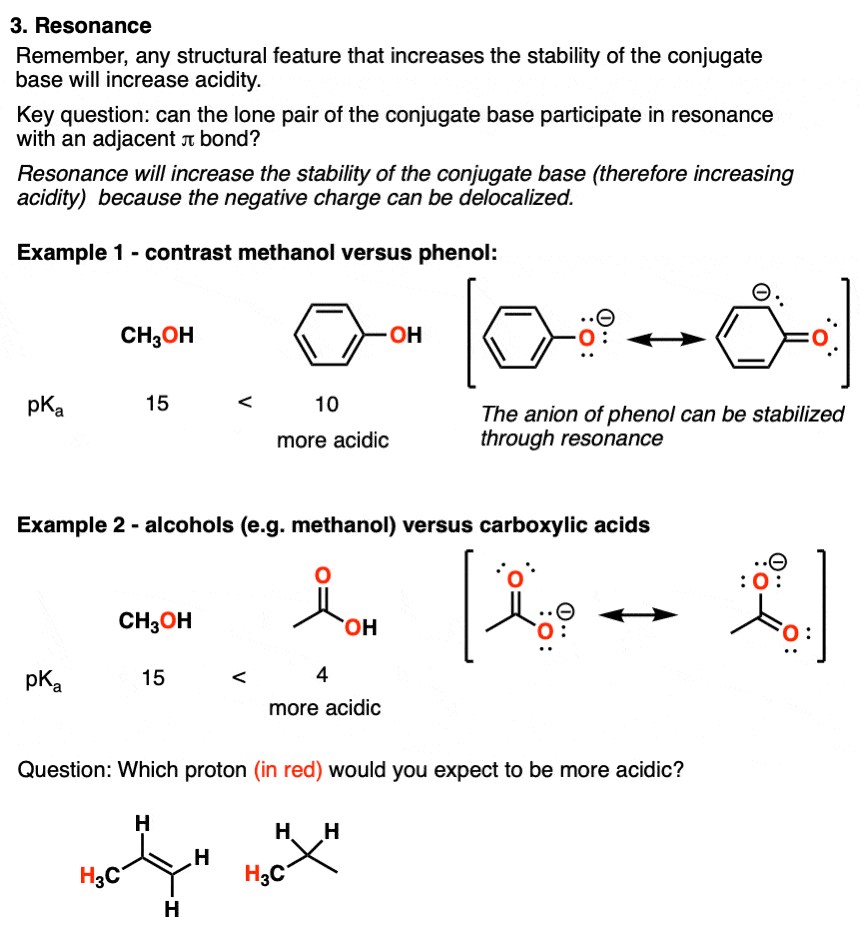
2.3. Resonance: Delocalization of Charge
Resonance is a crucial factor in stabilizing the conjugate base. When the negative charge of the conjugate base can be delocalized over multiple atoms through resonance, the stability of the base increases, leading to a stronger acid.
Examples:
- Phenol (C6H5OH) vs. Ethanol (CH3CH2OH): Phenol is significantly more acidic than ethanol because the negative charge on the phenoxide ion (the conjugate base of phenol) can be delocalized around the benzene ring through resonance. This delocalization stabilizes the phenoxide ion, making phenol a stronger acid.
- Acetic Acid (CH3COOH) vs. Ethanol (CH3CH2OH): Acetic acid is more acidic than ethanol because the negative charge on the acetate ion (the conjugate base of acetic acid) can be delocalized over both oxygen atoms through resonance.
Table 3: Acidity Trends Based on Resonance
| Compound | Conjugate Base | Resonance Stabilization | pKa | Relative Acidity |
|---|---|---|---|---|
| Ethanol | Ethoxide (CH3CH2O-) | None | 16 | Low |
| Phenol | Phenoxide (C6H5O-) | Yes | 10 | High |
| Acetic Acid | Acetate (CH3COO-) | Yes | 4.76 | High |
2.4. Dipole Induction: The Impact of Electronegative Atoms
Inductive effects arise from the presence of electronegative atoms or groups near the acidic proton. These electronegative groups pull electron density towards themselves, which stabilizes the conjugate base by dispersing the negative charge. The strength of the inductive effect depends on two main factors:
- Electronegativity: More electronegative atoms exert a stronger inductive effect.
- Distance: The inductive effect decreases with increasing distance between the electronegative atom and the negative charge.
Examples:
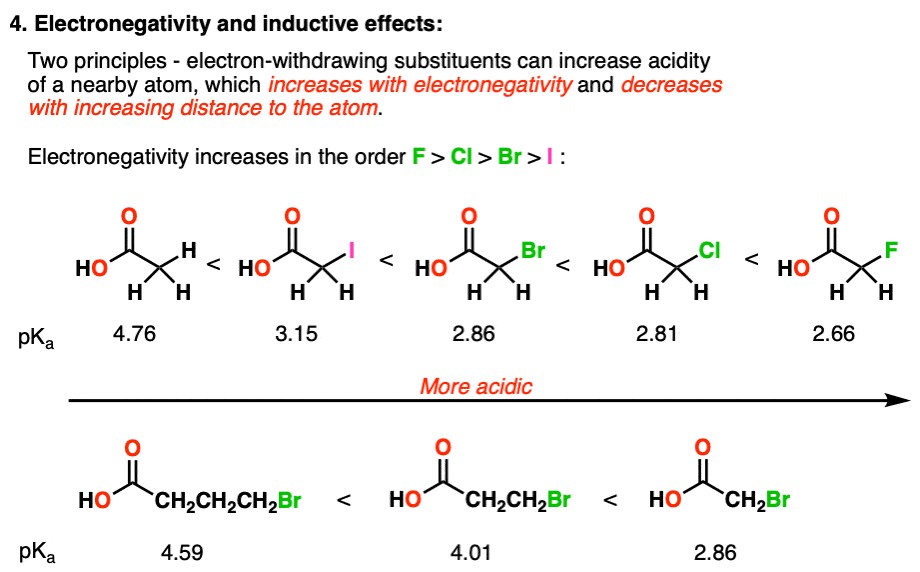
- Trifluoroacetic Acid (CF3COOH) vs. Acetic Acid (CH3COOH): Trifluoroacetic acid is a much stronger acid than acetic acid. The three fluorine atoms, which are highly electronegative, pull electron density away from the carboxylate group, stabilizing the negative charge on the conjugate base.
- Chloroacetic Acid (ClCH2COOH) vs. Acetic Acid (CH3COOH): Chloroacetic acid is more acidic than acetic acid due to the inductive effect of the chlorine atom.
Table 4: Acidity Trends Based on Inductive Effects
| Compound | Electronegative Atoms/Groups | Distance from Acidic Site | pKa | Relative Acidity |
|---|---|---|---|---|
| Acetic Acid | None | N/A | 4.76 | Low |
| Chloroacetic Acid | Cl | Close | 2.87 | Moderate |
| Trifluoroacetic Acid | F (3) | Close | 0.23 | High |
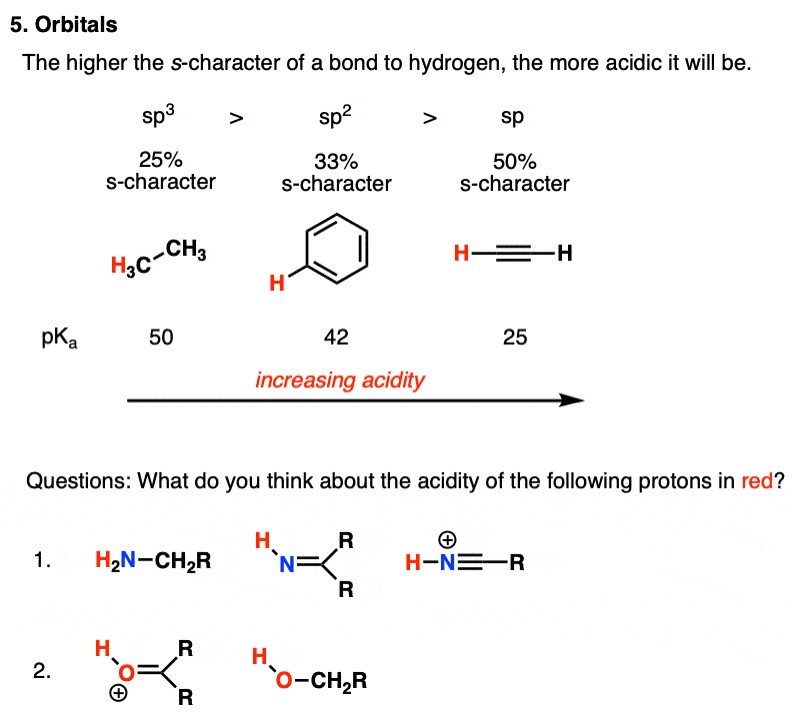
2.5. Orbitals: Hybridization and Acidity
The type of orbital that holds the lone pair of electrons in the conjugate base also affects acidity. Acidity increases with increasing s-character in the orbital. This is because s orbitals are closer to the nucleus than p orbitals, so electrons in an s orbital are more stable.
Examples:
- Alkynes (sp hybridized C-H) vs. Alkenes (sp2 hybridized C-H) vs. Alkanes (sp3 hybridized C-H): Terminal alkynes are more acidic than alkenes, which are more acidic than alkanes. This is because the sp hybridized carbon in alkynes has 50% s-character, the sp2 hybridized carbon in alkenes has 33% s-character, and the sp3 hybridized carbon in alkanes has 25% s-character.
Table 5: Acidity Trends Based on Orbitals
| Compound | Hybridization | s-Character | pKa | Relative Acidity |
|---|---|---|---|---|
| Alkane | sp3 | 25% | ~50 | Low |
| Alkene | sp2 | 33% | ~44 | Moderate |
| Alkyne | sp | 50% | ~25 | High |
3. Applying CARDIO: Examples and Applications
Understanding the CARDIO principles is essential, but applying them to real-world examples is where your understanding truly solidifies. Let’s explore some scenarios where we use CARDIO to compare the acidity of organic compounds.
3.1. Comparing Acidity of Alcohols and Carboxylic Acids
Consider ethanol (CH3CH2OH) and acetic acid (CH3COOH). Which one is more acidic? Using CARDIO:
- Charge: Both are neutral, so charge doesn’t play a significant role.
- Atom: Both have the acidic proton attached to an oxygen atom.
- Resonance: Acetic acid’s conjugate base (acetate ion) is stabilized by resonance, whereas ethanol’s conjugate base (ethoxide ion) is not.
- Dipole Induction: While both have electronegative oxygen atoms, the effect is more pronounced in acetic acid due to the carbonyl group.
- Orbitals: Not a primary factor in this comparison.
Based on these considerations, resonance stabilization in the acetate ion makes acetic acid much more acidic than ethanol.
3.2. Comparing Acidity of Different Phenols
Consider phenol (C6H5OH) and a phenol substituted with a nitro group (NO2) at the para position. Which is more acidic?
- Charge: Both are neutral.
- Atom: Both have the acidic proton attached to an oxygen atom.
- Resonance: Both have resonance stabilization in their conjugate bases. However, the nitro-substituted phenol has additional stabilization.
- Dipole Induction: The nitro group is highly electronegative and further stabilizes the negative charge through inductive effects and resonance.
- Orbitals: Not a primary factor.
The nitro group’s additional resonance and inductive stabilization make the nitro-substituted phenol more acidic than unsubstituted phenol.
3.3. Predicting Acidity Trends in Substituted Benzoic Acids
Substituted benzoic acids provide a great platform for applying CARDIO principles. Consider benzoic acid (C6H5COOH) with different substituents:
- Electron-Donating Groups (e.g., -OCH3, -CH3): These groups destabilize the negative charge on the carboxylate anion, making the substituted benzoic acid less acidic than benzoic acid itself.
- Electron-Withdrawing Groups (e.g., -Cl, -NO2): These groups stabilize the negative charge on the carboxylate anion, making the substituted benzoic acid more acidic than benzoic acid itself.
Table 6: Acidity Trends in Substituted Benzoic Acids
| Substituent | Electronic Effect | Effect on Acidity |
|---|---|---|
| -H | None | Baseline |
| -OCH3 | Electron-Donating | Decreases |
| -CH3 | Electron-Donating | Decreases |
| -Cl | Electron-Withdrawing | Increases |
| -NO2 | Electron-Withdrawing | Increases |
4. Advanced Concepts in Acidity
Beyond the basic CARDIO principles, several advanced concepts can further refine your understanding of acidity.
4.1. Tautomerism and Acidity
Tautomerism is the ability of certain molecules to exist in two or more interconvertible forms that differ in the location of a proton and a double bond. Keto-enol tautomerism is a common example. The relative acidity of different tautomers can influence the overall acidity of a compound.
Example:
- Acetylacetone: This molecule can exist in both keto and enol forms. The enol form is stabilized by intramolecular hydrogen bonding, which can affect its acidity.
4.2. Steric Effects on Acidity
Steric effects refer to the spatial arrangement of atoms in a molecule. Bulky groups near the acidic proton can hinder solvation of the conjugate base, reducing its stability and, consequently, the acidity of the acid.
Example:
- Substituted Phenols: Bulky substituents ortho to the hydroxyl group can prevent the phenoxide ion from being properly solvated, decreasing the acidity.
4.3. Solvent Effects on Acidity
The solvent in which a reaction occurs can also influence acidity. Polar protic solvents (e.g., water, alcohols) can stabilize charged species through solvation, while polar aprotic solvents (e.g., DMSO, acetone) have a weaker ability to solvate ions.
Example:
- Acid-Base Reactions in DMSO: In DMSO, the acidity of weak acids can be significantly enhanced because DMSO is a poor hydrogen bond donor and does not effectively solvate anions.
5. Practical Tips for Comparing Acidity
Comparing acidity can be challenging, but with these practical tips, you can improve your skills:
- Master the CARDIO Principles: Understand each factor and its influence on acidity.
- Draw Resonance Structures: Always draw resonance structures to assess the stability of the conjugate base.
- Consider Inductive Effects: Pay attention to the presence of electronegative atoms and their distance from the acidic site.
- Look for Steric Hindrance: Bulky groups can impact the stability of the conjugate base.
- Practice, Practice, Practice: Work through as many examples as possible to reinforce your understanding.
6. Common Mistakes to Avoid
When comparing acidity, it’s easy to fall into common traps. Here are some mistakes to avoid:
- Ignoring Resonance: Failing to consider resonance stabilization is a frequent error.
- Overemphasizing Electronegativity: While electronegativity is important, it’s not the only factor.
- Neglecting Inductive Effects: Overlooking the influence of electronegative groups can lead to incorrect predictions.
- Not Considering All Factors: Acidity is a result of multiple factors, not just one.
7. Real-World Applications of Acidity Comparisons
Understanding acidity is not just an academic exercise. It has numerous real-world applications in chemistry, biology, and medicine.
7.1. Organic Synthesis
In organic synthesis, acidity plays a crucial role in designing reaction mechanisms and predicting reaction outcomes. For instance, understanding the acidity of different functional groups is essential for selecting appropriate reagents and conditions for reactions like deprotonations, enolate formation, and protecting group strategies.
7.2. Biochemistry
In biochemistry, acidity is vital in enzyme catalysis, protein folding, and drug design. The acidity of amino acid side chains, for example, influences the structure and function of proteins.
7.3. Drug Development
In drug development, the acidity of drug molecules affects their bioavailability, binding affinity, and metabolism. Understanding the pKa values of ionizable groups in drug molecules is critical for optimizing their pharmaceutical properties.
8. COMPARE.EDU.VN: Your Resource for Chemistry Comparisons
At COMPARE.EDU.VN, we are dedicated to providing comprehensive and reliable comparisons across a wide range of subjects, including chemistry. Whether you’re a student grappling with organic chemistry concepts or a professional seeking to deepen your understanding, our platform offers the resources you need to succeed.
8.1. Why Choose COMPARE.EDU.VN for Chemistry Comparisons?
- Detailed Analysis: We provide in-depth comparisons of chemical properties, including acidity, using the CARDIO principles and other advanced concepts.
- Real-World Examples: Our comparisons include real-world examples to help you apply your knowledge in practical situations.
- Expert Insights: Our content is developed by experts in the field to ensure accuracy and relevance.
- User-Friendly Interface: Our platform is designed to be easy to navigate, so you can quickly find the information you need.
8.2. How COMPARE.EDU.VN Can Help You
- Learn Key Concepts: Use our comparisons to master the fundamental principles of acidity and other chemical concepts.
- Prepare for Exams: Our detailed analysis and examples can help you prepare for exams and quizzes.
- Enhance Your Understanding: Deepen your understanding of chemistry by exploring our advanced topics and real-world applications.
- Make Informed Decisions: Whether you’re choosing reagents for a reaction or designing a drug molecule, our comparisons can help you make informed decisions.
9. Frequently Asked Questions (FAQ)
-
What is pKa, and why is it important?
pKa is a measure of acid strength. It is the negative logarithm of the acid dissociation constant (Ka). Lower pKa values indicate stronger acids.
-
How does resonance affect acidity?
Resonance stabilizes the conjugate base by delocalizing the negative charge over multiple atoms, which increases the acidity of the corresponding acid.
-
What is the inductive effect, and how does it influence acidity?
The inductive effect is the electron-withdrawing or electron-donating effect of substituents on a molecule. Electron-withdrawing groups stabilize the conjugate base, increasing acidity, while electron-donating groups destabilize it, decreasing acidity.
-
How does the hybridization of an atom affect acidity?
Acidity increases with increasing s-character in the orbital. sp hybridized carbons are more acidic than sp2 hybridized carbons, which are more acidic than sp3 hybridized carbons.
-
What role does electronegativity play in determining acidity?
Across a row of the periodic table, acidity increases with increasing electronegativity because more electronegative atoms can better stabilize the negative charge of the conjugate base.
-
How does atomic size influence acidity?
Down a group of the periodic table, acidity increases with increasing atomic size because the negative charge of the conjugate base is distributed over a larger volume, leading to greater stability.
-
Can steric effects influence acidity?
Yes, bulky groups near the acidic proton can hinder solvation of the conjugate base, reducing its stability and, consequently, the acidity of the acid.
-
How do electron-donating groups affect acidity?
Electron-donating groups destabilize the negative charge on the conjugate base, making the corresponding acid less acidic.
-
How do electron-withdrawing groups affect acidity?
Electron-withdrawing groups stabilize the negative charge on the conjugate base, making the corresponding acid more acidic.
-
Why is it important to consider multiple factors when comparing acidity?
Acidity is influenced by multiple factors, including charge, atom type, resonance, inductive effects, and orbitals. Considering all these factors provides a more accurate assessment of acidity.
10. Conclusion: Mastering Acidity Comparisons
Comparing the acidity of organic compounds requires a thorough understanding of the key factors that influence acid strength. By mastering the CARDIO principles—Charge, Atom, Resonance, Dipole Induction, and Orbitals—you can confidently predict and compare the acidic strength of different molecules. Whether you’re a student, researcher, or professional, a solid grasp of acidity is essential for success in chemistry and related fields.
Remember, COMPARE.EDU.VN is here to support you on your learning journey. We offer detailed comparisons, real-world examples, and expert insights to help you master chemistry concepts and make informed decisions.
Ready to take your understanding of acidity to the next level? Visit COMPARE.EDU.VN today to explore our comprehensive comparisons and resources. Make informed decisions with confidence and ease. Our team of experts is dedicated to providing you with the most accurate and up-to-date information. Contact us at 333 Comparison Plaza, Choice City, CA 90210, United States. Or reach out via Whatsapp at +1 (626) 555-9090. Start comparing now at compare.edu.vn and unlock your full potential in chemistry.