Lewis acid strength dictates chemical reactivity. COMPARE.EDU.VN offers comprehensive insights to understand and compare it effectively. This guide explores essential factors, aiding informed decisions. Explore related concepts like acid-base interactions and electron affinity.
1. Introduction to Lewis Acids and Bases
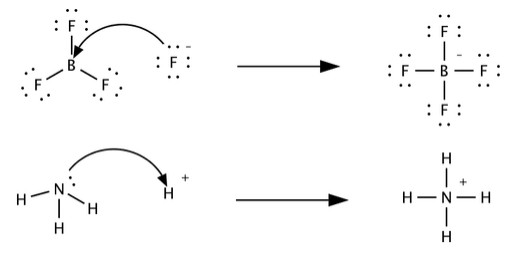
Understanding How To Compare Lewis Acid Strength requires a solid grasp of what Lewis acids and bases are. Unlike Brønsted-Lowry acids, which donate protons, Lewis acids are electron-pair acceptors, and Lewis bases are electron-pair donors. This broader definition allows for a wider range of chemical species to be classified as acids and bases.
1.1 The Lewis Definition
G.N. Lewis proposed this theory in 1923, shifting the focus from proton transfer to electron-pair sharing. This expanded the scope of acid-base chemistry beyond aqueous solutions. Lewis acids can accept an electron pair to form a covalent bond, while Lewis bases can donate an electron pair to form a covalent bond.
1.2 Examples of Lewis Acids and Bases
Common examples of Lewis acids include boron trifluoride (BF3), aluminum chloride (AlCl3), and metal cations like Fe3+. These species have vacant orbitals or positive charges that attract electron pairs. Lewis bases include ammonia (NH3), water (H2O), and halide ions like Cl-. They possess lone pairs of electrons that can be donated to form coordinate covalent bonds.
2. Factors Influencing Lewis Acid Strength
Several factors determine the strength of a Lewis acid. Understanding these factors is critical for comparing the acidity of different compounds.
2.1 Charge Density
A higher positive charge and smaller ionic radius result in greater charge density, enhancing the Lewis acidity of a metal ion. For instance, Al3+ is a stronger Lewis acid than Na+ due to its higher charge and smaller size. Charge density directly affects the electrostatic attraction between the Lewis acid and the electron-pair donor.
2.2 Electronegativity
The electronegativity of the central atom in a Lewis acid influences its ability to attract electrons. More electronegative elements tend to be stronger Lewis acids. This is because they can better stabilize the negative charge associated with accepting an electron pair.
2.3 Size and Steric Effects
Smaller Lewis acids can form stronger interactions with Lewis bases because the electron pair is closer to the positive charge. However, steric hindrance can also play a role. Bulky substituents around the Lewis acid center can prevent the approach of a Lewis base, reducing its effectiveness.
2.4 Empty Orbitals
The presence of accessible empty orbitals is crucial for Lewis acidity. Compounds with empty p or d orbitals, such as BF3 and transition metal ions, can readily accept electron pairs. The energy level of these empty orbitals relative to the energy level of the electron pair in the Lewis base also affects the strength of the interaction.
3. Comparing Lewis Acid Strength: Qualitative Analysis
Qualitative analysis involves comparing Lewis acid strengths based on general trends and properties.
3.1 Periodic Trends
Across a period, Lewis acidity generally increases with increasing electronegativity and decreasing atomic size. Down a group, Lewis acidity typically decreases due to increasing atomic size and decreasing charge density. Understanding these periodic trends provides a valuable framework for predicting relative acidities.
3.2 Inductive Effects
Electron-withdrawing groups increase the Lewis acidity of a compound by enhancing the positive charge on the central atom. Conversely, electron-donating groups decrease Lewis acidity. These inductive effects can significantly alter the acidity of organic and inorganic Lewis acids.
3.3 Resonance Effects
Resonance can stabilize the Lewis acid-base complex, increasing the overall acidity. If the resulting complex has resonance structures that delocalize the charge, the interaction will be stronger. This is particularly important in organic Lewis acids and bases.
4. Quantitative Measures of Lewis Acidity
Quantitative measures provide numerical values to compare Lewis acid strengths more precisely.
4.1 Gutmann Acceptor Number (AN)
The Gutmann Acceptor Number (AN) is a quantitative measure of Lewis acidity based on the molar enthalpy of the reaction between a Lewis acid and a reference Lewis base, usually triethylphosphine oxide (Et3PO), in a dilute solution of 1,2-dichloroethane. A higher AN value indicates a stronger Lewis acid.
4.1.1 How AN is Determined
The AN value is determined by measuring the chemical shift of the 31P NMR signal of Et3PO upon complexation with the Lewis acid. The larger the chemical shift, the stronger the interaction between the Lewis acid and Et3PO, and the higher the AN value.
4.1.2 Applications of AN
AN values are useful for comparing the Lewis acidity of different solvents and solutes. They can also be used to predict the outcome of chemical reactions involving Lewis acids and bases.
4.2 Drago-Wayland Equation
The Drago-Wayland equation is another quantitative method used to estimate the strength of Lewis acid-base interactions. This equation relates the enthalpy of adduct formation to parameters associated with the acid (EA) and base (EB), as well as parameters associated with the covalent (CA, CB) and electrostatic (AA, AB) contributions to the bond.
4.2.1 Equation Components
The Drago-Wayland equation is expressed as:
ΔH = EA EB + CA CB
Where:
- ΔH is the enthalpy change of the adduct formation.
- EA and EB are parameters characteristic of the acid and base, respectively, reflecting their ability to form electrostatic bonds.
- CA and CB are parameters characteristic of the acid and base, respectively, reflecting their ability to form covalent bonds.
4.2.2 Using the Equation
By determining these parameters experimentally, the strength of various Lewis acid-base interactions can be compared quantitatively. This approach is particularly useful for understanding the nature of bonding in coordination complexes and other adducts.
4.3 Computational Chemistry
Computational methods, such as density functional theory (DFT), can be used to calculate the interaction energies between Lewis acids and bases. These calculations provide valuable insights into the electronic structure and bonding characteristics of the resulting complexes.
4.3.1 DFT Calculations
DFT calculations can predict the geometry, stability, and electronic properties of Lewis acid-base adducts. The calculated interaction energies can be used to rank the relative strengths of different Lewis acids.
4.3.2 Advantages of Computational Methods
Computational methods offer several advantages, including the ability to study systems that are difficult to access experimentally and to provide detailed information about the bonding interactions.
5. Examples of Comparing Lewis Acid Strength
To illustrate how to compare Lewis acid strength, let’s consider several examples.
5.1 Comparison of Boron Halides
The boron halides (BF3, BCl3, BBr3, BI3) are a classic example of Lewis acids. The Lewis acidity of these compounds increases in the order BF3 < BCl3 < BBr3 < BI3. This trend is due to the increasing size of the halide atoms, which reduces the π-bonding between the boron and halide atoms, making the boron atom more electron-deficient and thus a stronger Lewis acid.
5.2 Comparison of Metal Cations
Metal cations such as Al3+, Fe3+, and Zn2+ are common Lewis acids. The Lewis acidity of these cations depends on their charge density and electronegativity. For example, Al3+ is a stronger Lewis acid than Zn2+ because it has a higher charge-to-size ratio.
5.3 Comparison of Organometallic Compounds
Organometallic compounds, such as Grignard reagents (RMgX) and alkylaluminum compounds (R3Al), are also Lewis acids. The Lewis acidity of these compounds depends on the nature of the metal and the substituents. Electron-withdrawing substituents increase the Lewis acidity, while electron-donating substituents decrease it.
6. Applications of Lewis Acidity in Chemistry
Understanding Lewis acidity is crucial in various areas of chemistry, including catalysis, organic synthesis, and materials science.
6.1 Catalysis
Lewis acids are widely used as catalysts in organic reactions. They can activate substrates by coordinating to electron-rich centers, facilitating reactions such as Friedel-Crafts alkylations and acylations, Diels-Alder reactions, and polymerization reactions.
6.2 Organic Synthesis
In organic synthesis, Lewis acids are used to promote a variety of transformations, including electrophilic additions, nucleophilic substitutions, and rearrangements. They can also be used to protect functional groups and to control the stereoselectivity of reactions.
6.3 Materials Science
Lewis acids play an important role in materials science, particularly in the synthesis of coordination polymers and metal-organic frameworks (MOFs). These materials have a wide range of applications, including gas storage, catalysis, and sensing.
7. Challenges and Limitations
While the concept of Lewis acidity is powerful, there are some challenges and limitations to consider.
7.1 Solvent Effects
The strength of a Lewis acid can be influenced by the solvent in which it is dissolved. Polar solvents can solvate Lewis acids, reducing their ability to interact with Lewis bases. Nonpolar solvents, on the other hand, can enhance the Lewis acidity.
7.2 Steric Hindrance
Steric hindrance can prevent the approach of a Lewis base, reducing the effectiveness of the Lewis acid. Bulky substituents around the Lewis acid center can create a steric barrier, making it difficult for the Lewis base to coordinate.
7.3 Hard and Soft Acid-Base Theory (HSAB)
The Hard and Soft Acid-Base (HSAB) theory provides a framework for understanding the selectivity of Lewis acid-base interactions. Hard acids prefer to interact with hard bases, while soft acids prefer to interact with soft bases. This theory can be used to predict the outcome of reactions involving Lewis acids and bases.
7.4 Ambiguity in Measurement
Quantitative measurement of Lewis acidity can be challenging, and different methods may give different results. The choice of method depends on the specific system being studied and the type of information required.
8. Recent Advances in Lewis Acid Chemistry
Recent advances in Lewis acid chemistry have led to the development of new catalysts, materials, and synthetic methods.
8.1 Chiral Lewis Acids
Chiral Lewis acids are used to catalyze enantioselective reactions, allowing for the synthesis of chiral molecules with high enantiomeric excess. These catalysts have been used in a wide range of asymmetric transformations, including Diels-Alder reactions, aldol reactions, and epoxidations.
8.2 Brønsted Acid-Assisted Lewis Acids (BLAs)
Brønsted acid-assisted Lewis acids (BLAs) combine the properties of Brønsted acids and Lewis acids to achieve synergistic catalysis. These catalysts can activate substrates through both proton transfer and electron-pair donation, leading to enhanced reactivity and selectivity.
8.3 Lewis Acidic Metal-Organic Frameworks (MOFs)
Lewis acidic metal-organic frameworks (MOFs) are a new class of materials that combine the high surface area of MOFs with the catalytic activity of Lewis acids. These materials have been used in a variety of catalytic applications, including gas-phase reactions, liquid-phase reactions, and photocatalysis.
9. Case Studies: Comparing Lewis Acid Strength in Real-World Applications
To further illustrate the practical applications of comparing Lewis acid strength, let’s explore several case studies.
9.1 Friedel-Crafts Alkylation
In Friedel-Crafts alkylation, Lewis acids such as AlCl3 are used to activate alkyl halides, generating electrophiles that react with aromatic compounds. The strength of the Lewis acid affects the rate and selectivity of the reaction. Stronger Lewis acids, such as AlCl3, can catalyze the reaction at lower temperatures and with higher yields compared to weaker Lewis acids like FeCl3.
9.2 Diels-Alder Reactions
Lewis acids are also used as catalysts in Diels-Alder reactions to lower the activation energy and increase the reaction rate. For instance, the use of a Lewis acid catalyst such as Eu(fod)3 (tris(6,6,7,7,8,8,8-heptafluoro-2,2-dimethyl-3,5-octanedionato)europium(III)) can significantly enhance the rate and stereoselectivity of the reaction.
9.3 Polymerization Reactions
Lewis acids are employed in polymerization reactions to initiate and control the polymerization process. For example, in cationic polymerization, Lewis acids such as BF3 or AlCl3 are used to initiate the polymerization of alkenes by generating carbocations. The strength of the Lewis acid influences the molecular weight and microstructure of the resulting polymer.
10. Future Directions in Lewis Acid Chemistry
The field of Lewis acid chemistry continues to evolve, with ongoing research focused on developing new catalysts, materials, and synthetic methods.
10.1 Development of New Lewis Acid Catalysts
Researchers are actively working on designing new Lewis acid catalysts with improved activity, selectivity, and stability. This includes the development of chiral Lewis acids for enantioselective reactions, Brønsted acid-assisted Lewis acids for synergistic catalysis, and Lewis acidic MOFs for heterogeneous catalysis.
10.2 Applications in Green Chemistry
There is growing interest in using Lewis acids in green chemistry applications, such as the development of more sustainable and environmentally friendly chemical processes. This includes the use of Lewis acids as catalysts in reactions that use renewable feedstocks, reduce waste, and minimize the use of toxic solvents.
10.3 Integration with Machine Learning
Machine learning is being increasingly used to predict and optimize the properties of Lewis acids and their catalytic activity. Machine learning models can be trained on large datasets of experimental and computational data to identify correlations between the structure and properties of Lewis acids, allowing for the rational design of new catalysts.
11. Conclusion
Comparing Lewis acid strength is essential for understanding and predicting chemical reactivity. By considering factors such as charge density, electronegativity, size, and orbital availability, and by using quantitative measures such as Gutmann Acceptor Numbers and computational methods, one can effectively compare the acidity of different compounds. Lewis acids have broad applications in catalysis, organic synthesis, and materials science, and ongoing research continues to expand the scope of Lewis acid chemistry.
Navigate the complexities of chemical comparisons effortlessly at COMPARE.EDU.VN, your ultimate resource for making informed decisions in the world of science and education.
Address: 333 Comparison Plaza, Choice City, CA 90210, United States.
Whatsapp: +1 (626) 555-9090.
Website: COMPARE.EDU.VN
12. FAQ: Understanding Lewis Acid Strength
12.1 What is a Lewis acid?
A Lewis acid is a chemical species that can accept an electron pair to form a covalent bond.
12.2 How does Lewis acidity differ from Brønsted acidity?
Brønsted acids donate protons, while Lewis acids accept electron pairs. Lewis acidity is a broader concept that encompasses many more substances than Brønsted acidity.
12.3 What factors influence the strength of a Lewis acid?
Factors include charge density, electronegativity, size, and the availability of empty orbitals.
12.4 How can I compare the Lewis acidity of different compounds?
You can use qualitative methods based on periodic trends and inductive effects, or quantitative methods such as Gutmann Acceptor Numbers and computational chemistry.
12.5 What is the Gutmann Acceptor Number (AN)?
The Gutmann Acceptor Number (AN) is a quantitative measure of Lewis acidity based on the molar enthalpy of the reaction between a Lewis acid and triethylphosphine oxide.
12.6 What are the applications of Lewis acids in chemistry?
Lewis acids are used as catalysts in organic reactions, to promote various transformations in organic synthesis, and in materials science for the synthesis of coordination polymers and metal-organic frameworks.
12.7 What are the limitations of using Lewis acids?
Limitations include solvent effects, steric hindrance, and the complexity of hard and soft acid-base interactions.
12.8 What are chiral Lewis acids?
Chiral Lewis acids are Lewis acids that contain chiral ligands, allowing them to catalyze enantioselective reactions.
12.9 What are Brønsted acid-assisted Lewis acids (BLAs)?
Brønsted acid-assisted Lewis acids (BLAs) combine the properties of Brønsted acids and Lewis acids to achieve synergistic catalysis.
12.10 How are Lewis acids used in materials science?
Lewis acids are used in the synthesis of coordination polymers and metal-organic frameworks (MOFs), which have applications in gas storage, catalysis, and sensing.
Dive deeper into chemical comparisons! Visit compare.edu.vn for detailed analysis and make smarter, informed decisions.