Introduction
In various aspects of life, comparisons are fundamental to understanding differences and making informed decisions. Just as we might compare heights, such as 5’9 compared to 5’2, to appreciate a visual difference, in the realm of health and medicine, comparing the effectiveness of different interventions is crucial. In the United States, for instance, two primary types of influenza vaccines are available, each with its own administration method and target demographic: the intranasal Live Attenuated Influenza Vaccine (LAIV) and the injectable Trivalent Inactivated Vaccine (TIV). Understanding their relative efficacy is akin to understanding the tangible difference between 5’9 compared to 5’2 – it’s about discerning which option provides superior protection, particularly for different populations.
The availability of both LAIV and TIV offers valuable choices, accommodating individual preferences regarding administration and perceived side effects. However, beyond mere availability, the crucial objective is to guide individuals toward the vaccine that offers optimal protection against influenza, much like choosing the right tool for a specific job based on comparative effectiveness. The efficacy of these vaccines can fluctuate based on the age of the recipient and other variables. This article delves into recent studies comparing the efficacy of LAIV and TIV in both children and adults, aiming to provide insights for healthcare providers and policymakers in formulating optimal vaccination strategies, ensuring everyone gets the ‘taller’ protection they need against influenza.
Relative Efficacy of LAIV and TIV in Children (6 Months–18 Years): A Heightened Defense
When considering protection against influenza in children, the landscape of vaccine efficacy reveals a clear distinction, much like the noticeable height difference between 5’9 compared to 5’2. Studies directly comparing LAIV and TIV in children consistently point towards LAIV as the more protective option.
Direct Comparisons of LAIV and TIV Against Natural Influenza Infection
Initial evaluations of LAIV and TIV in children were conducted in Europe and Israel during 2002–2003 (Figure 1, Table 1). Ashkenazi et al. (2011) in a multinational study involving 2187 children aged 6–71 months with recurrent respiratory tract infections, compared two doses of LAIV or TIV. The results showed a significant 53% reduction in culture-confirmed influenza cases caused by vaccine-matched strains among LAIV recipients compared to TIV recipients. This is a substantial difference, analogous to the visual impact of 5’9 compared to 5’2. Furthermore, LAIV recipients also experienced fewer healthcare visits and missed fewer school days due to all-cause respiratory illnesses. Interestingly, even when influenza occurred in LAIV recipients, the severity was notably reduced.
Figure 1.
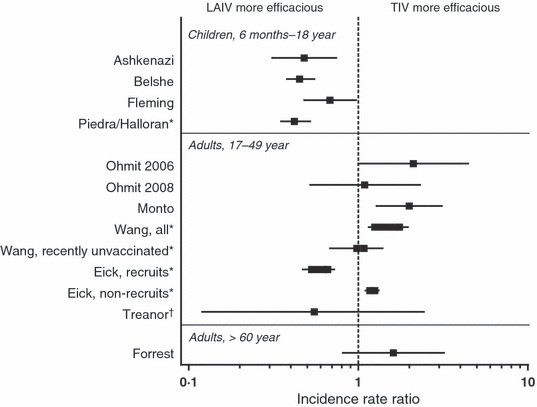 Figure 1
Figure 1
Alt text: Incidence Rate Ratios comparing LAIV and TIV vaccine efficacy in children and adults, highlighting differences in protection levels.
Fleming et al. (2011) in a separate study in 2229 children aged 6–17 years with asthma, found that LAIV recipients had 35% fewer influenza cases caused by matched strains compared to TIV recipients. While the difference in other health outcomes wasn’t as pronounced as in the Ashkenazi study, the trend towards better protection with LAIV remained consistent.
Belshe et al. (2011) conducted a large double-blind study with 8352 children aged 6–59 months, comparing LAIV and TIV. The study revealed a 45% reduction in matched strain influenza and a remarkable 58% reduction in mismatched strain influenza in LAIV recipients compared to TIV. This broader protection against both matched and mismatched strains further underscores the superior efficacy of LAIV in young children, akin to having a larger safety margin when considering 5’9 compared to 5’2 in a safety-critical application. Similar to the Ashkenazi study, breakthrough illnesses were less severe in LAIV recipients.
Table 1.
Summary of studies comparing LAIV and TIV efficacy in children and adults, showing varying degrees of protection.
| Study, Year | Subject age | Sample size | Design | Endpoint | Incidence regardless of antigenic match (LAIV versus TIV) | Predominant Strains | Absolute Efficacy LAIV, % (95% CI) | Absolute Efficacy TIV, % (95% CI) | Incidence Rate Ratio (LAIV/TIV) (95% CI) | Illness Severity in Vaccinated Individuals |
|---|---|---|---|---|---|---|---|---|---|---|
| Children 6 months to 18 years of age | ||||||||||
| Ashkenazi et al., 2002–2003 | 6–71 months | 2187 | Prospective, randomized, open‐label | CCI | 2·8% versus 5·8% | Matched B | NA | NA | 0·48 (0·31, 0·74) | Less fever and fewer missed school/daycare days among LAIV breakthrough cases, with trend toward less antibiotic use |
| Belshe et al., 2004–2005 | 6–59 months | 8352 | Prospective, randomized, double‐blind | Culture‐confirmed modified CDC‐ILI | 3·9% versus 8·6% | Mismatched A/H3N2 | NA | NA | 0·45 (0·38, 0·55) | Less fever among LAIV breakthrough cases |
| Fleming et al., 2002–2003 | 6–17 years | 2229 | Prospective, randomized, open‐label | CCI | 4·5% versus 6·6% | Matched B | NA | NA | 0·68 (0·48, 0·97) | No differences seen |
| Piedra/Halloran et al., 2003–2004 | 5–18 years | 6403 | Prospective, non‐randomized, open‐label | MAARI | 2·81 versus 6·64 per 1000 child‐days | Mismatched A/H3N2 | 56 (32, 75) | No effectiveness observed | 0·42 (0·35, 0·52)† | Not reported |
| Adults 17–49 years of age | ||||||||||
| Ohmit et al., 2004–2005 | 18–46years | 1247 | Prospective, randomized, open‐label* | Culture‐ or PCR‐confirmed illness | 4·0% versus 1·9% | Mismatched A/H3N2 | 48 (−7, 74) | 75 (42, 90) | 2·11 (1·00, 4·44) | Not reported |
| Ohmit et al., 2005–2006 | 18–48 years | 2058 | Prospective, randomized, open‐label* | Culture‐ or PCR‐confirmed illness | 1·6% versus 1·5% | Matched A/H3N2 | 8 (−194, 67) | 16 (−171, 70) | 1·09 (0·52, 2·31) | Not reported |
| Monto et al., 2007–2008 | 18–49 years | 1952 | Prospective, randomized, open‐label* | Culture‐ or PCR‐confirmed illness | 6·9% versus 3·4% | Matched A/H3N2 | 36 (0, 59) | 68 (46, 81) | 2·00 (1·28, 3·11) | Not reported |
| Wang et al., 2004–2007†,‡ | 17–49 years | >1 million per season | Retrospective, non‐randomized | ICD‐9 code for pneumonia or influenza | NA | Not reported | NA | NA | All: 1·25–1·75 (1·15, 1·96) Recently unvaccinated: 0·98–1·08 (0·68, 1·39) | NA |
| Eick et al., 2005–2007†,‡,§ | 17–49 years | >750 000 per season | Retrospective, non‐randomized | ICD‐9 code for ILI | NA | Not reported | NA | NA | Recruits: 0·53–0·66 (0·47, 0·69) Non‐recruits: 1·17–1·25 (1·15, 1·27) | NA |
| Challenge study: Treanor et al., 1995–1996 | 18–45 years | 92 | Prospective, randomized, double‐blind, wild‐type challenge | CCI | 6·9% versus 12·5% | Matched A/H1N1, A/H3N2, B | 85 (28, 100) | 71 (2, 97) | 0·55 (0·12, 2·43) | Trend toward less severe symptoms among LAIV vaccinees |
| Adults ≥60 years of age | ||||||||||
| Forrest et al., | >60 years | 3009 | Prospective, randomized, ouble‐blind | CCI | 1·4% versus 0·9% | Matched A/H1N1, matched A/H3N2, mismatched B | NA | NA | 1·61 (0·81, 3·20) | Trend toward less feverishness and fever among LAIV breakthrough cases |
Alt text: Table summarizing comparative studies of LAIV and TIV effectiveness across different age groups and study designs.
LAIV and TIV Against Influenza-Like Illness: Broadening the Scope
Piedra et al. (2011) conducted a large non-randomized trial in the US during the 2003–2004 season, a period dominated by the mismatched A/Fujian/411/02 (H3N2) virus. This study demonstrated LAIV’s effectiveness in preventing medically attended acute respiratory illness in children aged 5–18 years, with an estimated efficacy of 56%. Notably, TIV showed no effectiveness during this period, highlighting LAIV’s advantage against mismatched strains. This is a crucial finding, suggesting LAIV provides a ‘taller’, more robust defense, especially when the influenza strains are not perfectly matched to the vaccine, much like how 5’9 compared to 5’2 offers more reach in unpredictable situations. However, it’s important to note that direct LAIV-TIV comparison in this study should be interpreted cautiously due to the non-randomized nature of the groups.
Relative Efficacy in Adults (17–49 Years): A More Level Playing Field?
In adults aged 17–49 years, the comparative efficacy of LAIV and TIV presents a more nuanced picture, less like the stark contrast of 5’9 compared to 5’2 and more like comparing two individuals of similar height with slight advantages in different areas.
Experimental Challenge Studies
Treanor et al. (2011) conducted an early placebo-controlled challenge study in 92 adults aged 18–45 years. When challenged with wild-type influenza viruses, LAIV showed an 85% protective efficacy and TIV showed 71%. While LAIV numerically outperformed TIV, the difference was not statistically significant. However, there was a trend towards less severe illness in LAIV recipients, hinting at a potential qualitative advantage, even if not a statistically taller barrier against infection.
Natural Infection Studies: Varied Terrain
Multi-year studies by University of Michigan researchers (Monto et al., 2011; Ohmit et al., 2011, 2012) compared LAIV and TIV in healthy adults aged 18–49 years across three influenza seasons. In the 2004–2005 season, dominated by a drifted A/H3N2 strain, TIV appeared more efficacious than LAIV. In 2005–2006, with low influenza activity, the efficacy of both vaccines was similar and statistically insignificant. In 2007–2008, again with a drifted A/H3N2 strain, TIV again showed superior efficacy to LAIV. These findings suggest that in young adults, particularly against drifted strains, TIV might offer a ‘taller’ protective barrier in some seasons.
Influenza-Like Illness Studies in Military Settings
US military databases provide a unique setting for large-scale observational studies. Wang et al. (2011) in a retrospective study of over a million non-recruit service members, found that TIV vaccination was associated with a lower incidence of pneumonia or influenza compared to LAIV. However, Eick et al. (2011), in a subsequent analysis, differentiated between recruits and non-recruits. Interestingly, in military recruits, LAIV was more protective against influenza-like illness, while in non-recruits, TIV was again more effective. This divergence suggests that in populations with different levels of prior immunity and exposure (recruits being a more immunologically naive and densely populated group), the relative efficacy of LAIV and TIV can vary significantly. For recruits, LAIV provided a ‘taller’ defense, while for non-recruits, TIV seemed to stand ‘taller’.
Placebo-Controlled Studies from 1997-1998: Historical Perspective
Two placebo-controlled studies from 1997–1998, one for LAIV and one for TIV, offer an additional comparative point. During a season with a mismatched A/H3N2 strain, LAIV showed effectiveness in reducing febrile illness, while TIV showed no effectiveness. This historical data point again underscores LAIV’s potential advantage against mismatched strains in adults, echoing findings in children and contrasting with some later studies that favored TIV in certain adult populations.
Efficacy in Older Adults (≥60 Years): Approaching Similar Heights
In adults aged 60 and above, the data on LAIV and TIV efficacy is more limited, and suggests a convergence in effectiveness, where the height difference between 5’9 compared to 5’2, so apparent in other contexts, becomes less relevant in terms of vaccine efficacy.
Direct Comparison and Placebo-Controlled Studies
A direct comparative study of LAIV and TIV in older adults by Forrest et al. (2011) was inconclusive due to low influenza incidence. However, placebo-controlled studies of LAIV and TIV in older adults suggest similar levels of efficacy against matched strains. While these studies have limitations, the overall trend indicates that in older adults, both vaccines offer comparable protection, reaching a similar ‘height’ of efficacy.
Combination Studies: Exploring Synergies
Studies exploring the combination of LAIV and TIV in older adults have shown some promise. Employed together, they have demonstrated improved protection against influenza A compared to TIV alone in nursing home residents. However, in COPD patients, the added benefit of LAIV to TIV was less pronounced. These combination studies suggest potential avenues for enhancing protection in vulnerable older populations, though more research is needed.
Safety Profiles: Side-by-Side Comparison
Safety is paramount when comparing medical interventions. Prospective studies comparing LAIV and TIV generally indicate comparable safety profiles in individuals aged 2 years and older. Most adverse effects are mild and transient for both vaccines.
LAIV is associated with increased runny nose and nasal congestion across age groups, and may cause low-grade fever and decreased appetite in young children, and sore throat, cough, and headache in adults. TIV is linked to injection site reactions and, less frequently, fever, muscle aches, and oculorespiratory syndrome in adults.
A notable safety concern with LAIV emerged in children under 24 months, where an increased risk of medically significant wheezing and hospitalization was observed in one study. This led to the contraindication of LAIV in this age group. In older children and adults, however, safety profiles are largely similar, and both vaccines are generally well-tolerated.
Conclusions: Weighing the Heights of Protection
In conclusion, both LAIV and TIV are effective tools for preventing influenza. In children, LAIV consistently demonstrates superior protection against influenza compared to TIV across multiple studies and against both matched and mismatched strains. This advantage is akin to the clear height advantage of 5’9 compared to 5’2 in many situations.
In adults aged 17–49 years, the relative efficacy is more variable. While some studies suggest TIV may be more efficacious, particularly against drifted strains in some populations, others, especially those from earlier years and in military recruits, indicate LAIV’s potential advantage, especially against mismatched strains. The context, population, and specific influenza season appear to play crucial roles in determining which vaccine stands ‘taller’ in this age group.
In older adults aged 60 and above, current data suggests LAIV and TIV offer similar levels of protection. The ‘height’ of efficacy appears to converge in this age group.
The choice between LAIV and TIV should consider these age-specific efficacy profiles, individual preferences, and contraindications. For children, LAIV generally appears to be the ‘taller’ choice in terms of efficacy. For adults, the decision is more nuanced and may depend on factors such as age, health status, and circulating influenza strains. Continued research, particularly in adult populations and against varying severities of influenza illness, is crucial to further refine our understanding and optimize influenza vaccination strategies, ensuring everyone receives the ‘heightened’ protection they need.
Acknowledgments
The authors extend their gratitude to Xionghua Wu, PhD, and Gregory Susla, PharmD, and Jay Bauman, PharmD, for their contributions to data analysis and compilation. Editorial assistance was provided by Complete Healthcare Communications, Inc., funded by MedImmune.